We have made much progress in improving care, quality, and outcomes for children with cardiovascular disease across the world. Over the past decade, global health strategies have been initiated, including the following:
-
∙ Development of global clinical databases to facilitate data collection.
-
∙ Development of risk-adjustment to support outcome assessment.
-
∙ Identification of quality metrics to benchmark individual centre performance.
-
∙ A commitment to using improvement science methodologies.
These efforts have advanced the collective expertise and performance of inter-professional healthcare teams caring for children with cardiovascular disease worldwide. In this section, we highlight selected quality improvement initiatives and strategies affecting the field of cardiovascular care and describe implications for future practice and research.
International Quality Improvement Collaborative for Congenital Heart Surgery in Developing World Countries (IQIC)
During the 2007 Global Forum on Humanitarian Medicine in Cardiology and Cardiac Surgery in Geneva, Switzerland, clinical leaders discussed the global burden of CHD and the need for benchmarking outcome data in evolving world congenital heart surgery programmes. Representatives from Boston Children’s Hospital and non-governmental organisations joined forces to form a quality improvement collaborative focused on evaluating performance and driving improvement efforts for the care of CHD in resource-limited settings. The IQIC seeks to reduce mortality and major complications for children with CHD undergoing surgery or catheterisations. Led by an international steering committee and managed at Boston Children’s Hospital, the IQIC facilitates and empowers a collaborative of healthcare teams around the world to create a culture of patient safety and quality.
Since its inception in 2008, IQIC has grown from five to 58 sites in 24 countries. IQIC began with the establishment of a web-based congenital heart surgical database. De-identified data are entered for all surgical patients under 18 years of age, focusing on nutritional status, prematurity, age, surgical procedure, co-morbidities, and outcomes such as mortality and infections. Annual visits are conducted by Boston Children’s Hospital, non-governmental organisations partners, or virtually, to audit 10% of key data elements to ensure validity and review the IQIC goals. In 2016, 93% of sites passed data verification and were included in the annual benchmarking report. These reports are an opportunity for teams to determine their individual progress in improving outcomes and to benchmark centre performance in resource-limited settings.
The IQIC database has over 62,000 cases and uses the risk adjustment for congenital heart disease (RACHS-1) methodology to examine centre performance across participating sites.Reference Jenkins, Castañeda and Cherian 1 In 2015, among 10,728 cases, 5.5% were operated on ⩽30 days of age, 39.8% were operated on 31 days to <1 year, and 52.6% 1–17 years. In all, 5.8% were premature and 2.8% had major non-cardiac structural anomalies. Cases were categorised as RACHS-1: 15.7% Category 1, 48.9% Category 2, 24.4% Category 3, 6.7% Category 4, and 0.7% Categories 5–6. The overall in-hospital mortality rate was 4.9%, bacterial sepsis rate was 3.5%, and surgical site infection rate was 1.7%. Overall, risk-adjusted mortality and risk-adjusted infections decreased over time.Reference Jenkins, Castañeda and Cherian 1 , Reference Sen, Morrow and Balachandran 2
In 2010, a quality improvement effort to further reduce mortality and morbidities was instituted. Key drivers included safe perioperative practices, reduction of surgical site infections and bacterial sepsis, team-based practice, and nursing empowerment and education (Fig 1). Monthly webinars were facilitated by Boston Children’s Hospital highlighting the IQIC key drivers. These presentations have been translated into multiple languages and restructured to fit the needs of sites. The IQIC has also held a series of international learning sessions throughout 2013–2017. These inter-professional meetings are designed to enhance learning and sharing of each other’s work.

Figure 1 International Quality Improvement Collaborative for Congenital Heart Surgery in Developing World Countries Key Driver Diagram.
In 2017, the IQIC added a new key driver of nutritional management. Also new is the development of a catheterisation registry to examine outcomes for interventional procedures, and quality improvement activities related to interventional catheterisation and intraoperative perfusion. Data collection focused on the resources needed to perform cardiac surgery in low-resource settings is underway. The IQIC collaborative is free to join and may be contacted at internationalqi@childrens.harvard.edu.
United States quality improvement collaboratives in cardiac critical care
Quality improvement collaboratives in the United States represent one of the most effective approaches for improving patient outcomes. The Northern New England Cardiovascular Disease Study GroupReference O’Connor, Plume and Morton 3 and the Michigan Blue Cross/Blue Shield collaborativesReference Share, Campbell and Birkmeyer 4 have both demonstrated reduced surgical morbidity and mortality among adult patients. The core principles of collaborative quality improvement illustrated by these successful programmes include the following:
-
∙ Purposeful collection of granular clinical data.
-
∙ Timely feedback of clinician performance.
-
∙ Continuous quality improvement using empirical data and collaborative learning.
Increasingly, transparency between hospitals has proven to be a very effective method for promoting quality improvement efforts.Reference Forrest, Margolis, Seid and Colletti 5 , Reference Lihn, Kugler, Peterson, Lannon, Pickles and Beekman 6 Collaborative learning approaches in congenital cardiac care have used these principles focusing on perioperative and critical care. The National Pediatric Cardiology Quality Improvement Collaborative has demonstrated improved weight gain and lower mortality during the inter-stage period for children undergoing stage 1 palliation for hypoplastic left heart syndrome and related diagnoses.Reference Anderson, Beekman and Kugler 7 , Reference Anderson, Beekman and Kugler 8 Mahle et al from the NHLBI Pediatric Heart Network used a collaborative learning intervention to reduce the length of mechanical ventilation after repair of tetralogy of Fallot and coarctation in infants.Reference Mahle, Nicolson and Hollenbeck-Pringle 9 , Reference Wolf, Lee and Nicolson 10
The Consortium of Congenital Cardiac Care Measurement of Nursing Practice (C4-MNP) is a nurse-led collaborative committed to developing and evaluating paediatric cardiovascular nurse-sensitive quality indicators to improve and standardise nursing practices.Reference Connor, Mott, Green, Larson and Hickey 11 , Reference Connor, Larson, Baird and Hickey 12 Established in 2011, the consortium is a national community of nurse leaders, clinical experts, and nurse scientists committed to rigorous measurement of nursing care to achieve optimal outcomes for children with cardiac disease.Reference Connor, Mott, Green, Larson and Hickey 11 The C4-MNP has developed evidenced-based nurse-sensitive measures to use for assessment and improvement in nursing practice across the 31 collaborative paediatric cardiovascular programmes.Reference Connor, Mott, Green, Larson and Hickey 11 – Reference O’Connell, Ziniel, Hartwell and Connor 13
The Pediatric Cardiac Critical Care Consortium (PC4; pc4quality.org) is a quality collaborative dedicated to improving care and outcomes for children and adults with critical congenital cardiovascular disease.Reference Gaies, Cooper and Tabbutt 14 Started in 2009 with funding from the National Institutes of Health, PC4 began with the launch of its clinical database. The effort has increased from six hospitals in 2013 to 28 hospitals submitting data across North America. PC4 aims to expand its membership to other continents and to disseminate its findings widely to improve care across the globe.
The granular PC4 cardiac critical care database powers a reporting platform displaying real-time benchmark data on clinical outcomes and resource utilisation metrics (Fig 2). Data collection for the PC4 clinical registry is harmonised with other clinical registries including the Society of Thoracic Surgeons Congenital Heart Surgery Database and the ACC IMPACT Database. PC4 uses a robust, on-site audit method to ensure data intergrity.Reference Gaies, Donohue and Willis 15
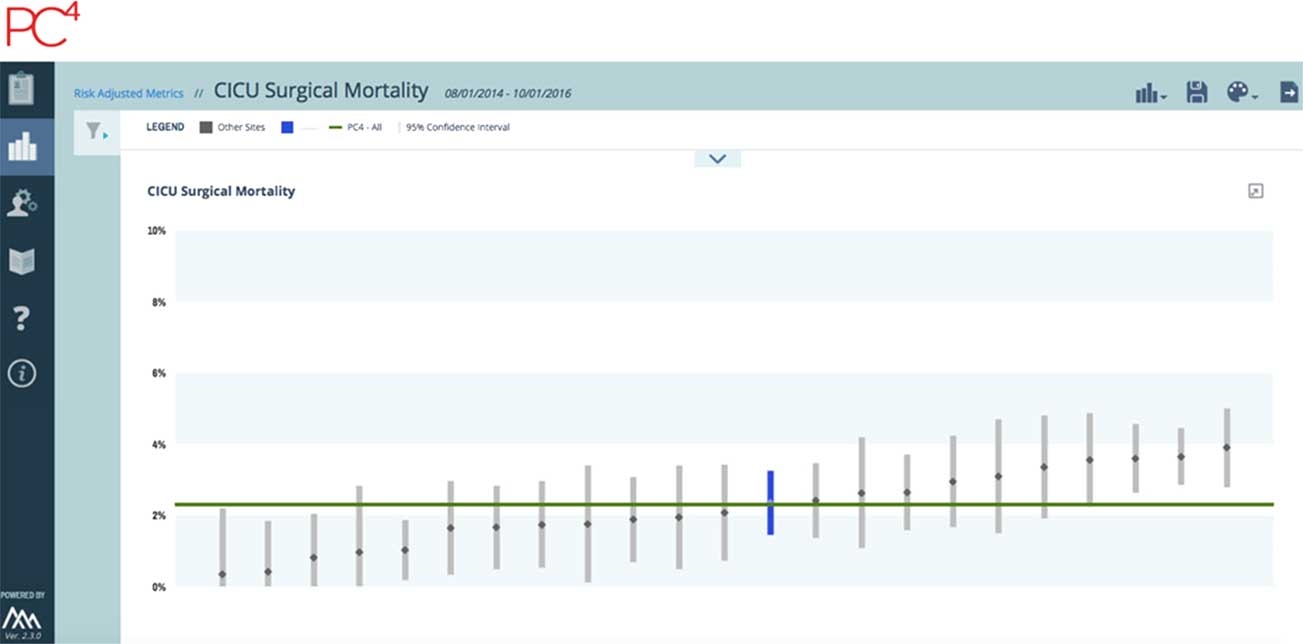
Figure 2 Example of real-time, adjusted benchmark reports for cardiac critical care units in Pediatric Cardiac Critical Care Consortium showing adjusted cardiac intensive care unit (CICU) mortality is shown in the figure. From pc4quality.org. Downloaded by M. Gaies, May 3, 2017.
A unique feature of PC4 is its commitment to transparency: hospitals can identify one another internally on the reporting platform by viewing outcome reports that are unblinded. The unblinding feature allows hospitals to recognise high performers, thus facilitating conversations between participating hospitals who desire to improve the quality of their care through reduction of complications or optimising cost-effectiveness and efficiencies. This level of transparency is a condition of participation in PC4. Teams routinely interact remotely and in-person to share practices and care protocols; champions from high-performing centres are given the opportunity to describe their approach to specific aspects of critical care in which their hospital is achieving excellent outcomes. In addition to providing the infrastructure for ad hoc local quality improvement efforts, PC4 has generated data suggesting opportunities for more far-reaching, multi-institutional collaborative learning projects. A collaborative-wide cardiac arrest prevention intervention is being implemented based on data showing wide variation in rates of cardiac arrest across PC4 cardiac ICUs.
In addition to providing a quality improvement infrastructure, PC4 maintains an active scientific portfolio. PC4 has primarily focused on two main domains of research: developing risk-adjustment methods to generate quality metrics for cardiac ICUs and surgical programmes, and describing variation in outcomes across cardiac ICUs. As an example, PC4 investigators performed an analysis to create a risk-adjustment model specifically designed to assess the quality of cardiac ICU postoperative care. This model, which is complementary to existing surgical risk-adjustment methods, incorporates postoperative illness-severity variables at the time of postoperative admission to the cardiac ICU in order to more accurately describe population risk at the time of transfer of care to the critical care team. Other early PC4 work has described variation in extubation failure rates across hospitals,Reference Gaies, Tabbutt and Schwartz 16 the epidemiology and centre variation in chylothorax rates,Reference Buckley, Graham and Gaies 17 and the development of novel case mix-adjusted duration of postoperative mechanical ventilation metrics.Reference Gaies, Werho and Ghanayem 18
These successful examples illustrate that the core concepts presented above can lead to important improvements in the care of critically ill children and adults with congenital cardiovascular disease: these collaborations collected detailed data on practice and outcomes, identified the highest-performing hospitals in their collaborative, disseminated this information back to participants, and identified the practices at high-performing hospitals that could be implemented at lower-performing hospitals to improve outcomes.
The World Database for Pediatric and Congenital Heart Surgery
“A Tool for Global Quality Improvement in Congenital Heart Surgery”
To satisfy its mission to “promote the highest quality of comprehensive cardiac care to all patients with congenital heart disease”, members of the World Society for Pediatric and Congenital Heart Surgery (WSPCHS) strategised, developed, and have now implemented a global database. The WSPCHS database is supported by the James and John Kirklin Institute for Research in Surgical Outcomes and is currently housed at the University of Alabama, Birmingham. The World Database for Pediatric and Congenital Heart Surgery (WDPCHS) went live on January 1, 2017, following identification of the appropriate variables to collect, creating a design for implementation on a global scale regardless of socio-economic status, and engaging in strategies to encourage international participation.Reference Kirklin and St. Louis 19
The World Database was developed to produce meaningful performance and quality analyses of surgical outcomes capturing important morbidities and mortalities for up to 1 year postoperatively. By using standardised terms and definitions developed and adjudicated by several national and international expert organisations, the database contains a global understanding, communication, and assessment of congenital cardiac practices.Reference Kirklin and St. Louis 19 To provide individual centres adequately detailed outcomes analyses while remaining cognizant of limited financial and personnel resources, the variables believed to provide the greatest opportunities to evaluate programmatic deficiencies and to effect necessary improvements in preoperative selection, intraoperative performance, and postoperative management were selected. Institutions will be able to confidentially compare their centre-specific data with regional, national, and international aggregate data. Table 1 illustrates the organisation and types of information that is being collected by the database. Institutional Practice data are collected in sufficient detail to allow for the creation of an international registry of congenital cardiac centres. Such information collected includes annual centre case volumes, the number of congenital cardiac surgeons, geographic region served, population served, the number of other institutions within its geographic region, and a description of the services provided. This information will allow not only for the ongoing assessment of congenital cardiac care on a global basis but will provide critical data to justify allocation of needed resources within specific regions. The surgery form requires the input of only 19 variables. These variables, including prior cardiac operations, preoperative risk factors, weight, cardiopulmonary bypass times, and intraoperative complications, were chosen by international experts in the field of congenital cardiac surgery and felt to reliably represent the operative conduct of a surgical programme. The collection of follow-up information on readmission, morbidities, and mortalities for up to 1 year postoperatively is a distinguishing feature that separates this database from others.
Table 1 World Database for Pediatric and Congenital Heart Surgery.

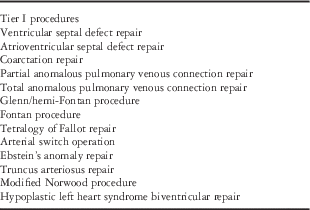
Data quality is critical, but collection of too many data may lead to the dilution of efficient analysis, increased expenditure of valuable resources, and loss of enthusiasm from participating members.Reference Vener, Gaies, Jacobs and Pasquali 20 , Reference Jacobs, Wernovsky and Elliott 21 For these reasons, surgical procedures are assigned to one of two categories within the database. This two-tiered system was created to allow for both the input of detailed data for a pre-selected group of surgical procedures and collection of a limited number of variables for all congenital cardiac procedures. Using the international prevalence and importance of specific CHD, a limited list of surgical procedures with the greatest power to ensure successful quality improvement initiatives were selected (Table 2). The information on surgical procedures includes data related to preoperative selection and intraoperative conduct. It further gathers follow-up information covering important morbidities, readmission for intervention related to the index surgical procedure, and mortality for up to 1 year postoperatively.
Table 2 World Database for Pediatric and Congenital Heart Surgery.
To facilitate participation in this database, the WSPCHS has provided this global quality improvement programme to all institutions that have an active congenital cardiac surgical programme free of charge. WSPCHS members from programmes that lack sufficient resources and facilities to undertake sophisticated outcomes analyses or whose country does not presently have a national database will be able to benchmark outcomes by comparing their analyses with national and international aggregate data. Participants will be blinded to individual programme outcomes other than their own. For members of the WSPCHS who are fortunate enough to possess the financial resources and manpower to use one of several national or intercontinental congenital databases – e.g., The European Congenital Heart Surgeons Association database, The Society of Thoracic Surgeons Congenital database, and the Japanese Society Congenital database – participation in the WDPCHS will provide critical benchmarking information and intellectual support on a global basis. To encourage participation of centres that already submit to these national databases, the WDPCHS has developed cross-maps that will allow for the input of files they currently submit. This will prevent the depletion of precious resources and duplication of effort, while supporting the mission of this society.
By establishing the World Database, the World Society believes it is taking an important step in the global improvement of care for children with CHD. In the longer term, it is anticipated that the greater numbers in a global database will allow for more accurate risk adjustment than is possible in national databases.
Fast-tracking pathways
Fast-tracking is a process in which patients are moved through the perioperative period in the most expeditious manner, balancing resource utilisation with patient safety and comfort.Reference Vener, Gaies, Jacobs and Pasquali 20 Time to extubation is one of the key components of this goal. Early extubation, very early extubation, and ultra-fast extubation have become common in both lower- and higher-resource environments. There is no consistent definition for these or similar terms – they may refer to patients whose endotracheal tube is removed at the conclusion of surgery, whereas other references include patients extubated up to 24 hours after arrival in the ICUs.Reference Wong, Lai, Chee and Lee 22 – Reference Silva, Cartacho, Castro, Salgado Filho and Brandao 25
Implementing these processes is multi-disciplinary, beginning preoperatively with patient selection, continuing with intraoperative events and anaesthetic decisions, and then into the ICU. For this to work effectively, all stakeholders need to understand and participate, including physicians, nurses, respiratory support, and other staff members.
The primary driver for fast-tracking is resource utilisation.Reference Chang, Lorenzo and Macario 26 In a low-resource environment, ventilators and other supplies may be limited; bringing a patient out of the operating room without the need for a ventilator may increase ICU throughput and decrease potential hazards associated with limited equipment. In countries without these constraints, fast-tracking protocols have the benefit of increasing patient safety and comfort, as well as the decreased equipment and personnel costs associated with ventilator management, medication expenses, and complications associated with prolonged mechanical ventilation. Fast-tracking also allows more rapid patient mobilisation, neurologic assessment, and patient-centred analgesia by facilitating patient communication and assessment.
Early extubation of paediatric cardiac surgical patients is not a new phenomenon. In its infancy, paediatric cardiac surgery was typically associated with intraoperative extubation because postoperative ventilators for children were unavailable.Reference Keats, Kurosu, Telford and Cooley 27 Its utility significantly waned with the development of better ventilators for children and publications that associated high-dose narcotic techniques and postoperative mechanical ventilation with improved cardiac surgical outcomes.Reference Anand and Hickey 28 However, successful reports from practitioners working in low resource countries on mission trips reinvigorated the concept.Reference Schechter, Navedo and Jordan 29
There are a variety of mechanisms to achieve the goal of early extubation, but all rely on limited intraoperative narcotic usage and adjunctive short-acting sedatives such as dexmedetomidine or propofol along with inhaled anaesthetics. Some physicians have advocated for long-acting neuraxial opioids to limit intravenous narcotic needs to facilitate early extubation.Reference Mittnacht, Thanjan and Srivastava 23 , Reference Garg, Rao and John 30 The specific techniques themselves are of less importance than adherence to a well-designed process and clear communication.
A recent systematic review and meta-analysis on early extubation in paediatric cardiac surgery by Alghamdi et al highlighted wide variation and poor statistical quality of the studies.Reference Alghamdi, Singh and Hamilton 31 A prospective randomised study on early extubation in paediatric cardiac patients included 100 consecutive patients ranging from infants to adolescents, whereas Neirotti et al described early extubation in over 1000 patients in a retrospective review and Garg et al described extubation in the operating room prospectively in 1000 patients.Reference Alghamdi, Singh and Hamilton 30 , Reference Preisman, Lembersky and Yusim 32 , Reference Neirotti, Jones, Hackbarth and Paxson Fosse 33 Despite certain limitations, all demonstrated that early extubation is achievable and feasible in neonatal patients undergoing complex cardiac repairs, albeit not at as high a rate as achieved in older patients.Reference Varghese, Kutty, Abdullah, Hall, Shostrom and Hammel 34
As shown in Tables 3 and 4, the risks of early extubation for congenital heart surgery patients can be mitigated by proper patient selection, appropriate analgesia, meticulous surgery with intraoperative assessment for significant residual lesions and haemostasis, well-conducted cardiopulmonary bypass, and strict monitoring in the postoperative ICU.
Table 3 Benefits and concerns associated with early extubation and fast-tracking.
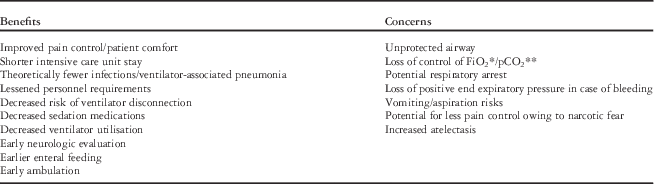
* Fraction of inspired oxygen
** Partial pressure of carbon dioxide
Table 4 Relative contraindications for early extubation.

* Trisomy 21 is commonly encountered and is not a direct contraindication, depending upon airway evaluation and ease of intubation
** PH is a relative contraindication. It is commonly found in developing countries in older patients with large shunts. Surgery may be the only therapeutic option and removing the endotracheal tube relieves a significant PH crisis stimulus
*** Although neonates may be placed on a fast-track protocol, they have a significantly higher incidence of reintubation as do patients who have longer bypass times or higher Society of Thoracic Surgeons – European Association for Cardio-Thoracic Surgery Congenital Heart Surgery Mortality Categories (STAT)/Risk Adjustment for Congenital Heart Surgery (RACHS-1) surgeries
Fast-tracking involves the entire care process beyond extubation and includes clinical pathways, starting from pre-anaesthetic assessment, preparation, admission through surgery, to admission into the ICU, that facilitate other related end points such as chest tube and line removal or transfer to non-ICU wards and discharge. Other items that may affect hospital stays include the availability of transportation home, outpatient pharmacy needs, and cardiology clinic follow-up.Reference Mahle, Jacobs and Jacobs 35
Fast-tracking is a paradigm shift to improve the quality of care while decreasing resource utilisation. Its development and implementation may strengthen clinical and non-clinical teams by improving communication.
Conclusions
The collaborative learning of inter-professional healthcare teams, implementation of quality initiatives, and rigorous evaluation has improved the global outcomes for congenital heart patients. As these and other efforts continue to evolve, there will be much opportunity to advance the practice and science of paediatric cardiovascular care. The continued leveraging of technology, commitment to data transparency, focus on team-based practice, and recognition of cultural norms and preferences ensure the success of sustainable models of global collaboration.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.








