Children with critical congenital heart disease (CHD) are known to be at risk for neurodevelopmental delay, including motor delays/impairments. Reference Wernovsky and Licht1,Reference Snookes, Gunn and Eldridge2 More than a decade ago Majnemer et al Reference Majnemer, Limperopoulos, Shevell, Rosenblatt, Rohlicek and Tchervenkov3 reported gross motor delays affected almost half of all school aged children who had survived open heart surgery. Holm et al found that children with critical CHD have a risk of severe motor impairment that is up to 11 times higher than those of same age healthy children; Reference Holm, Fredriksen, Fosdahl, Olstad and Vøllestad4 and recently a systematic review showed all children with CHD are at high risk of motor impartment from birth to adolescence. Reference Bolduc, Dionne, Gagnon, Rennick, Majnemer and Brossard-Racine5
Critical congenital heart disease can be characterised as biventricular or univentricular. In general, children with univentricular heart disease have been described to be at higher risk for neurodevelopmental impairments when compared with children with biventricular defects. Reference Dittrich, Bührer, Grimmer, Dittrich, Abdul-Khaliq and Lange6,Reference Creighton, Robertson and Sauve7
While previous studies have compared the motor outcomes of children with univentricular critical CHD with those of healthy controls, Reference Bjarnason-Wehrens, Dordel and Schickendantz8–Reference Sarajuuri, Lönnqvist and Mildh10 a comparison of the motor profile of preschool children with biventricular and univentricular critical CHD not diagnosed with cerebral palsy or acquired brain injury is yet to be completed.
This study aims to compare the frequency of motor impairment between preschool children with univentricular and biventricular critical CHD not diagnosed with cerebral palsy or acquired brain, to describe and compare the motor profiles of children with biventricular and univentricular critical CHD, and to explore possible predictors of motor impairment in each group.
Methods
This comparison study of two groups within an inception cohort is part of the multiprovincial Western Canadian Complex Pediatrics Therapies Follow Up program. This study was conducted in five different developmental/rehabilitation sites across Western Canada: Vancouver, British Columbia; Edmonton, Alberta; Regina and Saskatoon, Saskatchewan and Winnipeg, Manitoba. Reference Robertson, Sauve and Joffe11 All children registered in this follow-up program are identified at the time of their first cardiac surgery and followed prospectively. At the time of their initial cardiac surgery their demographic, pre-, intra-, and post-surgical information is collected. All surviving children undergo neurodevelopmental testing at pre-specified intervals. Specific details on this project have been previously reported. Reference Robertson, Sauve and Joffe11 This study was approved by the health research ethics board at each site. The parents or legal guardians of all participating children signed informed consent.
Participants
All children with critical CHD registered with the Western Canadian Complex Pediatrics Therapies Follow Up program who underwent complex cardiac surgery at ≤6 weeks of age at Stollery Childrens’ Hospital, Edmonton, Canada from 2009 to 2014, with the exception of those from the Calgary site, were eligible for inclusion. Children with an intellectual quotient <70 were excluded from the study, as lower scores in this group could represent difficulties with the ability to understand and follow the assessment’s directions and tasks.
Those with a confirmed chronic neuromotor disability (including cerebral palsy/acquired brain injury) Reference Ricci, Andersen and Joffe12 were also excluded from this study, as were children who died before the 4.5-year-old assessment or were lost to follow-up.
For the purpose of this study participating children were divided into two groups, those with univentricular critical CHD and those with biventricular critical CHD. In this study univentricular hearts included those defects with an anatomical single ventricle, and those with “functionally univentricular hearts”, representing essentially all lesions that require palliative surgery as they are not amenable for biventricular corrective surgical repair. Reference Frescura and Thiene13,Reference Khairy, Poirier and Mercier14 (Fig 1).

Figure 1. Flowchart of death, lost, excluded and assessed children after complex cardiac surgery at <6 weeks of age from the years 2009–2014.
Childhood clinical assessments
As part of this follow-up program, all registered children underwent multidisciplinary assessment with standardised measures at approximately 4.5 years of age. At the time of this assessment, motor skills, the primary outcome of this study, were assessed by a physiotherapist or an occupational therapist with the Movement Assessment Battery for Children-second edition Reference Henderson, Sugden and Bernett15 a standardised measure of motor competence for children aged 3–16 years using eight tasks that are combined under three categories: Manual Dexterity, Aiming and Catching, and Balance. Results for each category are expressed in standard scores that have a mean of 10 and a standard deviation of 3; low standard scores indicate a poor performance. The test also provides a total score with a percentile equivalent; total scores <5th percentile indicate a motor function impairment.
In addition, all children were assessed by a developmental paediatrician who determined the presence of chronic neuromotor disability based on the medical history and findings from the physical examination and neuroimaging. Cognitive abilities were assessed by a psychologist using the Wechsler Preschool and Primary Scales of Intelligence – Third Edition; Reference Wechsler16 adaptive abilities were determined through the parents completed Adaptive Behaviour Assessment System-2. 17
Socio-demographic variables included maternal education in years, and the Blishen Index Reference Blishen, Carroll and Moore18 an indicator of socioeconomic status based on employment, education, and prestige value of an occupation (population mean of 43 and SD of 13).
Acute care variables
Acute care information included birth gestation (weeks), birth weight (grams), gender, multiple birth, chromosomal abnormality, antenatal diagnosis, and first surgery preoperative ventilation days; preoperative and postoperative highest plasma lactate and inotrope score; Reference Wernovsky, Wypij and Jonas19 age (days), weight (kg), cardiopulmonary bypass time (minutes), X-clamp time (minutes), and use of deep hypothermic circulatory arrest at first cardiac surgery; the presence of pre- or postoperative sepsis, seizures, cardiopulmonary resuscitation, extra corporeal membrane oxygenation, dialysis; and the number of ventilated, intensive care unit, and hospital days. Overall events recorded were the number of cardiac surgeries with cardiopulmonary bypass and ventilation days, presence of sepsis, cardiopulmonary resuscitation, dialysis, extracorporeal membrane oxygenation, heart transplant, ventricular assist device support, and extracorporeal cardiopulmonary resuscitation for each child before the 4.5-year-assessment.
Statistical analysis
In this study categorical variables are presented as proportions and continuous variables are presented as means (standard deviation) or medians (inter quartile range). Frequency of motor impairment is given as percentage of assessed survivors. Student t-test and χ2 test were used to compare groups. Multiple logistic regression analysis was conducted for each group individually and included demographic, operative and perioperative predictors of motor impairment having p value of <0.10 after screening for multicollinearity. Results are expressed as odds ratios with 95% confidence interval; significance considered at <0.05. Data analyses were performed using IBM SPSS Statistic Data Editor v 22 (IBM Corporation, Armonk, New York).
Results
At a mean age of 55.4 (standard deviation 3.77) months, 119 (72% of those eligible for inclusion) children (85 (71.4%) with biventricular critical CHD; 34 (28.6%) with univentricular critical CHD, 66.7% male) underwent testing with the Movement Assessment Battery for Children-second edition.
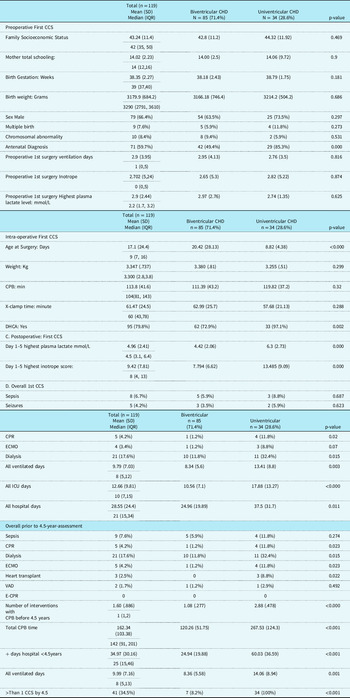
Table 1 describes the demographic, pre-, intra- and postoperative characteristics of children with biventricular and univentricular CHD. The growth, health and accompanying impairments of children with univentricular and biventricular critical CHD at time of testing are described in Table 2.
Table 1. Description of 4.5-Year-Old Children with biventricular and univentricular congenital heart disease n = 119: Mean (SD), Median (Interquartile Range), n (%)

Abbreviations: CCS = complex cardiac surgery; CPB = cardiopulmonary bypass; CPR = cardiopulmonary resuscitation; DHCA = Deep hypothermic circulatory arrest; ECMO = extracorporeal membrane oxygenation; E-CPR = extracorporeal- cardiopulmonary resuscitation; ICU = intensive care unit; IQR = interquartile range; SD = standard deviation; VAD = ventricular assist device.
Table 2. Growth, health, and accompanying impairments at 4.5 years (n = 119): Mean (sd), Median (Interquartile Range), n (%).

Abbreviations: ABAS = Adaptive behavior assessment system; GAC = general adaptive composite; IQ = intellectual quotient; IQR = interquartile range; SD = standard deviation.
Overall, 10/85 (11.8%) of children with biventricular critical CHD, and 11/34 (32.4%) (p = 0.008) of those with univentricular critical CHD had total Movement Assessment Battery for Children-second edition scores <5th percentile, representing motor function impairment. On average, total Movement Assessment Battery for Children-second edition scores of children with univentricular heart disease were 2.3 points lower than those of children with biventricular heart disease (6.44 (SD 2.8) versus 8.73 (2.9), p = <0.001). The comparison of the motor profiles (including Manual dexterity, Balance and Aiming, and catching scale scores) between children with univentricular and biventricular critical CHD is represented in Table 3.
Table 3. Comparison of motor profile as determined by the Movement Assessment battery for Children-second edition results in relation to Biventricular or Univentricular critical CHD, (mean) sd.

Independent odds ratios for motor impairment in children with biventricular critical CHD was presence of chromosomal abnormality (OR: 10.9, 95% CI 2.13–55.8) (p = 0.004). In children with univentricular critical CHD independent odds ratios were: postoperative day 1–5 highest lactate (mmol/L) at first complex cardiac surgery, (OR 1.65, 95% CI 1.04–2.62) (p = 0.034), and dialysis requirement any time before the 4.5-year-old assessment, (OR: 7.8, 95% CI 1.08–56.5) (p = 0.042).
Discussion
Results of this study indicate that among preschool survivors of critical CHD without cerebral palsy, acquired brain injury, or intellectual impairment, children with univentricular critical CHD are at higher risk of motor impairment when compared to those with biventricular critical CHD, with one third meeting the diagnostic criteria for motor function impairment. Our findings are consistent with studies reporting higher rates of motor delays in children requiring multiple palliative surgeries that is, commonly those with univentricular heart disease, compared to those undergoing a corrective surgery. Reference Mussatto, Hoffmann and Hoffman20 Moreover, our group and others have previously identified children with univentricular CHD as being at higher risk of neurocognitive delays when compared to those with a biventricular defect. Reference Creighton, Robertson and Sauve7,Reference Martin, Ricci and Atallah21
Prenatal abnormalities in brain volume, Reference Sethi, Tabbutt and Dimitropoulos22 in preoperative cerebral blood flow, Reference Cheng, Ferradal and Vyas23 the greater number of required surgeries and postoperative hospitalisations, as well as higher incidence of feeding difficulties Reference Davis, Davis and Cotman24 often leading to a high need for gastrostomy tube feedings Reference Ricci, Alton and Ross25 among children with univentricular critical CHD compared to those with biventricular critical CHD may all play a role in explaining the differences in motor function impairment observed between the two groups. Moreover, both prenatally and for up to several years postnatally, the developing brain in children with univentricular critical CHD is subjected to prolonged periods of significantly decreased oxygenation; chronic hypoxemia has been shown to alter neuronal and glial protein expression in the fetal brain. Reference Pearce26
In addition to physiologic factors, environmental factors such as parental anxiety and overprotective behaviors may also contribute to poorer motor outcomes in children with critical CHD, particularly for children with univentricular critical CHD who require repeated surgeries during infancy. Parental overprotectiveness may lead to reduced exposure to physical activity which, in turn, can affect motor performance. 27
Results of this study demonstrate that children with univentricular critical CHD experience more challenges with balance tasks than those with biventricular critical CHD and overall display scores that are significantly below what is expected in the general population. Balance is a key component of motor proficiency. It provides the necessary base to support the movement of the head, torso and limbs; stabilising the body and keeping it in balance are “prerequisites for adaptive control of movement”. Reference Adolph28 Difficulties with balance can not only limit the ability of a child to participate in sport related activities; studies suggest that balance skills are independently associated with spatial performance among 6-year-old children, Reference Frick and Möhring29 and with reading and mathematics academic achievement scores in elementary school children, potentially having far reaching impacts Reference Rizzuto and Knight30 Different movement education programmes targeting balance, including exergames and pedal less bikes have been proven to improve balance skills in children. Reference Demir and Akin31,Reference Shim, Davis, Newman, Abbey and Garafalo-Peterson32
Although the difference in manual dexterity scores between children with univentricular and biventricular critical CHD was not statistically significant, the manual dexterity score of those with univentricular critical CHD was substantially lower than that of the general preschool-age population (>1 SD below the norms). The co-existence of balance and manual dexterity challenges among children with univentricular critical CHD could be explained by the relationship between postural stability and manual control in children. Reference Flatters, Mushtaq, Hill, Holt, Wilkie and Mon-Williams33
Our study found that among children with univentricular critical CHD, higher lactate values in the first five postoperative days following the first cardiac surgery predicted motor impairments. Higher perioperative lactate values are known to be associated with a higher postoperative mortality and morbidity, Reference Munoz, Laussen, Palacio, Zienko, Piercey and Wessel34,Reference Maarslet, Moller, Dall, Hjortholm and Ravn35 and have been identified to predict mental and motor delays as well as chronic neuromotor disability among children with critical CHD. Reference Ricci, Andersen and Joffe12,Reference Freed, Robertson and Sauve36 In addition, requirement of dialysis any time before the 4.5-year-old assessment was found to be a strong predictor of motor impairment. Children with cyanotic critical CHD have been identified to be at risk for acute renal failure leading to the requirement of dialysis; Reference Baskin, Gulleroglu, Saygili, Aslamaci, Varan and Tokel37 and importantly an association between postoperative lactate and the need for dialysis was described by Maarslet and collaborators. Reference Maarslet, Moller, Dall, Hjortholm and Ravn35 According to a study by Warady et al, Reference Warady, Kriley, Lovell, Farrell and Hellerstein38 gross motor delays are common among children receiving peritoneal dialysis for end-stage renal disease. While in this study children did not receive dialysis for chronic renal disease, the requirement of dialysis among our cohort may be an indicator of the level of severity of illness. Moreover, increased illness severity could also indicate decrease chances for physical activity in these children.
Among children with biventricular critical CHD, the presence of a chromosomal abnormality with an intellectual quotient >70, was predictive of significantly poorer motor outcomes. This finding is consistent with previous studies that showed children with deletion 22q11.2 had significantly worse mental and psychomotor developmental index scores compared to those without deletion 22q11.2. Reference Atallah, Joffe and Robertson39
The importance of studying motor skills relies on the essential role these play on a child’s emotional, psychosocial, and overall development. Motor development is known to have a broad and profound impact on all other developmental domains. Development of a child’s mobility grants the child independence and self-assurance, thereby ensuring psycho-emotional stability. At preschool, development of cognitive and social skills such as sharing and cooperation are also highly dependent on a child’s ability to be mobile and actively participate in games and activities with peers. Reference Bjarnason-Wehrens, Dordel and Schickendantz8 Motor proficiency and physical activity act synergistically to mutually reinforce one another. Obesity, although not identified in our study, is a common comorbidity in children with CHD and therefore, concurrently identifying motor impairments and promoting physical activity early are critical. Reference Pinto, Marino and Wernovsky40
Our study has several limitations. Data on 25 children who underwent testing with the Movement Assessment Battery for Children second edition was incomplete and as such could not be included in this study. Due to the observational nature of this study, we cannot account for the effect of other unmeasured confounders. Finally, the lack of routine brain imaging is a limitation for this study. Although we excluded all children with a confirmed diagnosis of cerebral palsy and/or acquired brain injury who represented those with abnormal findings on neuroimaging resulting in chronic motor difficulties, the lack of routine neuroimaging in all children may have resulted in missed evidence of brain injury in some of our subjects. The strengths of this study include the prospective design and the large number of children followed in this study.
Conclusions
Guidelines have been established regarding evaluation and management of neurodevelopmental outcomes in children with CHD. Reference Marino, Lipkin and Newburger41 In particular, children with univentricular critical CHD and those with biventricular critical CHD with a chromosomal anomaly merit early, close and active surveillance of their motor development. Early identification of motor impairments and particularly balance and manual dexterity impairments, through standardised testing allows for optimisation of interventions and supports that can ultimately improve children’s motor skills and prevent future physical and psychosocial health problems.
Acknowledgements
We would like to thank the children and their parents for their willingness to attend developmental follow-up as well as all the team members of the Western Canadian Complex Pediatric Therapies.
Financial support
Financial support was provided as grants for 1996–1999 and 2014-2015 from the Glenrose Hospital Foundation-clinical Research Grant, and 2000–2006 by Alberta Health and Wellness, Government of Alberta. Ongoing support provided by the hospitals of the Western Canadian Complex Pediatric Therapies Follow-up Group: Stollery Children’s Hospital, Edmonton Alberta; Alberta Children’s Hospital, Calgary, Alberta; Winnipeg Children’s Hospital, Winnipeg, Manitoba; Kinsmen Children’s Centre and Royal University Hospital, Saskatoon, Saskatchewan; Regina General Hospital, Regina, Saskatchewan; British Columbia Children’s Hospital, Vancouver, British Columbia.
Conflict of interest
The authors have indicated they have no conflicts of interest to disclose.