Anorexia nervosa (AN) tends to affect adolescents and young women and is characterised by restricted eating and emaciation. The aetiology of AN is still unclear.Reference Kaye, Fudge and Paulus 1 However, recent research suggests abnormalities in reward and executive control as potential underlying biological mechanisms in the pathogenesis of AN.Reference Kaye, Fudge and Paulus 1
Neural correlates of reward processing in individuals suffering from AN seem to be particularly interesting, especially responses to food rewards. A recent review by Zhu et al Reference Zhu, Hu, Wang, Chen, Guo and Li 2 on fMRI studies concluded that emotional arousal (especially disgust) reflected by hyperactivation in, for example, the amygdala, the anterior cingulate cortex (ACC) and the caudate nucleus plays an important role in the processing of food information in AN. This is in line with the concept of increased food cue reactivity in AN patients.Reference Giel, Wittorf, Wolkenstein, Klingberg, Drimmer and Schonenberg 3 , Reference Sanders, Smeets, van Elburg, Danner, van Meer and Hoek 4 Hypoactivity in somatosensory regions suggests reduced physical stimulation by food in patients.Reference Zhu, Hu, Wang, Chen, Guo and Li 2 The majority of studies, however, involve visual presentation of pictorial food stimuli; neural correlates of taste processing are less commonly investigated in AN.
During the application of sucrose or water, recovered AN patients have been found to have less neural activation in the insula and ventral striatum to both conditions compared with controls, which was interpreted as less pronounced reward reactions in those recovered from AN.Reference Wagner, Aizenstein, Mazurkewicz, Fudge, Frank and Putnam 5 Further, when exposed to a pleasant chocolate drink during both hunger and satiety, it was found that AN patients had increased responses in the hunger condition in the right amygdala and left medial temporal gyrus, and in the inferior temporal gyrus (extrastriate body area) in the satiety condition compared with controls.Reference Vocks, Herpertz, Rosenberger, Senf and Gizewski 6 The authors report that this could reflect fear of weight gain and a mental focus on the body in AN patients. In a more recent study using a reward-conditioning task, which has been associated with brain dopamine reward circuits, currently ill AN patients showed increased responses in the anteroventral striatum, insula and prefrontal cortex compared with the opposite response in an obese group. Together, this research points to aberrant brain reward activity in AN and blunted reward responses in obesity.Reference Frank, Reynolds, Shott, Jappe, Yang and Tregellas 7
We have developed a paradigm involving the sight and taste of food stimuli which enables us to look at two aspects of food responses: anticipatory and consummatory. The former is reflecting the concept of ‘wanting’ and the latter the concept of ‘liking’.Reference Berridge 8 , Reference Berridge, Robinson and Aldridge 9 Affective neuroscience studies of ‘wanting’ and ‘liking’ have suggested that these psychological processes map onto distinct brain reward systems. For example, studies of pleasure identify hedonic impact in the ventral pallidum, nucleus accumbens and orbitofrontal cortex,Reference Wheeler and Carelli 10 – Reference Berridge and Kringelbach 13 whereas ‘wanting’ or incentive salience is mediated by neural systems that include mesolimbic dopamine projections from the ventral tegmental area to the ventral striatum.Reference Berridge 14 Furthermore, dopamine has been shown to be involved in the learning about rewards in prefrontal cortical regions such as the ACC and the orbitofrontal cortex.Reference Dayan and Balleine 15
Using our paradigm, we have previously shown that females recovered from AN have increased neural responses to the anticipation of food in the occipital cortex, ACC and caudate and increased ventral striatum, insula and putamen activity to its consummation, compared with controls. As these are areas known to be involved in the processing of anticipatory and consummatory responses to rewarding stimuli in general and taste stimuli specifically, we suggested that our results might reflect a neural biomarker of risk for AN.Reference Cowdrey, Park, Harmer and McCabe 16 However, as periods of malnutrition could lead to changes in the brain including microstructural alterations,Reference Yau, Bischoff-Grethe, Theilmann, Torres, Wagner and Kaye 17 our results may have been a consequence of recurrent AN rather than a cause. One way to resolve this issue is to study the neural response in people at increased risk of AN before the onset of illness. One reliable risk factor for AN is family heritance with genetic heritability accounting for 50–80% of the risk of developing an eating disorder.Reference Bulik, Sullivan, Tozzi, Furberg, Lichtenstein and Pedersen 18 In a large controlled family study, 3.4% of female family members of women with AN had developed AN themselves.Reference Strober, Freeman, Lampert, Diamond and Kaye 19 AN was four times more common in siblings of AN patients compared with siblings of control participants of a nation-wide register-based Danish study.Reference Steinhausen, Jakobsen, Helenius, Munk-Jorgensen and Strober 20
The present study therefore aimed to examine the neural response to food in those with a familial history of anorexia nervosa (family history) but with no personal history, compared to those with no family history (FH), to determine whether anticipatory and/or consummatory neural responses are possible trait markers for AN and not simply scars of starvation. Knowing whether anticipatory and/or consummatory responses to food stimuli are dysfunctional in those at risk of AN can help inform the development of psychological and pharmacological treatments that can directly target either both or one of the processes separately. Furthermore, we include both rewarding and aversive food stimuli to assess whether any aberrant neural responses to food in the FH group is valence specific.
We hypothesised that the FH group would have increased neural responses during both anticipatory and consummatory phases in areas such as the anterior cingulate, orbitofrontal cortex, and striatum similar to those found in our study with individuals recovered from AN and confirming that the neural response to food might be a biomarker for risk of AN.
Method
Participants
Fifteen women whose sisters met the DSM criteria 21 for AN and twenty-one controls were recruited for this study. Participants were recruited through advertisements both in the university and on social media sites asking, ‘Do you have a sister with anorexia nervosa?’ Ethics approval for the study was obtained from the Research Ethics Committee at the University of Reading. Written consent was obtained from all participants.
All participants underwent a screening process that involved a brief email screening checking that they had a sister with AN. Sisters then completed the Eating Disorder Diagnostic Scale standardised questionnaire.Reference Stice, Telch and Rizvi 22 We included participants where a diagnosis of clinical AN had been made by a general practitioner and/or psychiatrist and the symptoms described met criteria for AN. We did not attempt to assess the possible presence of other Axis I or II disorders in AN sisters. Study participants had a face-to-face assessment using the Structured Clinical Interview for DSM-IV.Reference First, Spitzer, Gibbon and Williams 23 All participants rated the following questionnaires: the Beck Depression Inventory-2nd Edition (BDI-II),Reference Beck, Steer and Brown 24 the Fawcett–Clark Pleasure Scale (FCPS),Reference Fawcett, Clark, Scheftner and Gibbons 25 the Snaith–Hamilton Pleasure Scale (SHAPS),Reference Snaith, Hamilton, Morley, Humayan, Hargreaves and Trigwell 26 the Ruminative Thought Style Questionnaire,Reference Brinker and Dozois 27 the Temporal Experience of Pleasure Scale (TEPS),Reference Gard, Gard, Kring and John 28 the Behavioural Inhibition/Behavioural Activation Scale (BIS/BAS),Reference Jorm, Christensen, Henderson, Jacomb, Korten and Rodgers 29 the Ruminative Response Scale for Eating DisordersReference Cowdrey and Park 30 and the Eating Attitudes Test (EAT)Reference Garner, Olmsted, Bohr and Garfinkel 31 approximately 1 week before scanning. The participants completed a chocolate questionnaire to measure liking, craving and frequency of eating chocolate.Reference Cowdrey, Park, Harmer and McCabe 16 Current body mass index (BMI) was also recorded for each participant. Furthermore, IQ was tested using the National Adult Reading Test (NART).Reference Nelson 32 Inclusion criteria for all participants included: (1) BMI between 18.5 and 25 kg/m2, (2) maintenance of a weight in the healthy range (defined by the World Health Organization) since menarche, (3) no use of psychoactive medications in the previous 12 months, (4) no lifetime history of any Axis 1 psychiatric disorder on the Structured Clinical Interview for DSM-IV. Participants in the control group had no first-degree relative with a current or past eating disorder diagnosis. General exclusion criteria for all participants included a history of head injury, neurological or other severe medical illness, pregnancy, and any contradictions to MRI. All participants were fluent in English, right-handed, had normal or corrected to normal vision, and were not taking medication except for the contraceptive pill. On the scan day, participants completed a visual analogue scale (VAS) measuring mood-related items including happiness, anxiety and disgust; they also completed the mood questionnaire Befindlichkeits Scale (Bf-SR).Reference Zerssen and Petermann 33 Participants were also asked to refrain from eating chocolate for 24 h prior to being scanned, which was checked verbally before testing. Finally participants were also asked to rate how hungry they were immediately before scanning on a VAS between 1 and 10 (Table DS2).
The task was adapted from our previous studyReference McCabe, Mishor, Cowen and Harmer 34 and similar to our recent study.Reference Dean, Horndasch, Giannopoulos and McCabe 35 It consisted of 40 trials and had 4 conditions based on the trial type (reward or aversive) and its level of difficulty (easy/hard). Trial type was cued by a visual stimulus (2 s – anticipatory phase) which indicated either to work to win the chocolate taste or to avoid the aversive taste. Difficulty was determined by the amount of effort required to complete the effort stage (easy = 24, hard = 45 button presses). This required volunteers to press a button as fast as possible (< 6 s) to move a bar towards the pleasant picture (reward) or away from the unpleasant picture (aversive), allowing enough time to complete easy trials but not hard ones. If the volunteers were successful on reward trials, they received the chocolate taste (5 s delivery – consummatory phase, then 2 s swallow cue) and if not, they received the tasteless solution. If the volunteers were successful on aversive trials, they received the tasteless solution and if not, they received the unpleasant taste (consummatory phase). A grey image (2 s) was presented at the end of each trial as a control for the cues. Each condition was repeated 10 times, chosen by random permutation. To sustain motivation, four trials (two reward/two aversive) were longer at 9 s each. Volunteers also rated ‘wanting’ and ‘pleasantness’ (+2 to −2) on a VAS (Fig. 1).
Stimuli
For the anticipation phase, we used either a picture of liquid chocolate (reward) or of a mouldy drink (aversive). The rewarding taste was a Belgian chocolate drink and the aversive taste was a combination of the chocolate drink mixed with beetroot juice, providing a similar texture and caloric content, but that was rated as unpleasant in valence. A tasteless solution (25×10−3mol/L KCl and 2.5×10−3mol/L NaHCO3 in distilled H2O) was used as a rinse between trials. This was subtracted from the effects of the other taste stimuli to allow somatosensory and mouth movement effects to be removed.Reference McCabe, Mishor, Cowen and Harmer 34 A picture of a grey box was presented at the end of each trial to use it as a control visual stimulus. Solutions were delivered through three Teflon tubes held together by a plastic mouthpiece and connected by a one-way syringe-activated check valve (Model 14044-5, World Precision Instruments, Inc.), allowing 0.5 mL of solution to be manually delivered.
fMRI scan
The experimental protocol consisted of an event-related interleaved design. A Siemens Magnetom Trio 3T whole body MRI scanner and a 12-channel head coil were used at the Centre for Integrative Neuroscience and Neurodynamics at the University of Reading. 3T-weighted echo planar imaging slices were acquired every 2 s (repetition time 2 s). Thirty-six axial slices with in-plane resolution of 3×3 mm and between-plane spacing of 3 mm were obtained. The matrix size was 64×64, and the field of view was 192×192 mm. Acquisition was carried out during the task performance yielding, on average, 1130 volumes in total. A whole-brain 3T-weighted echo planar imaging volume of the above dimensions and an anatomic T1 volume with axial plane slice thickness of 1 mm and in-plane resolution of 1×1 mm were also acquired.
fMRI analysis
Statistical parametric mapping (SPM8; http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) was used to analyse the imaging data as documented in our previous study.Reference Cowdrey, Park, Harmer and McCabe 16 Data were pre-processed using realignment, normalisation to the Montreal Neurological Institute (MNI) coordinate system and spatial smoothing with a 6-mm full-width-at-half-maximum Gaussian kernel and global scaling. The time series at each voxel was low-pass filtered with a haemodynamic response kernel. Time series non-sphericity at each voxel was estimated and corrected for, and a high-pass filter with a cut-off period of 128 s was applied.
In the single-event design, a general linear model was then applied to the time course of activation in which stimulus onsets were modelled as single-impulse response functions and then convolved with the canonical haemodynamic response function. Linear contrasts were defined to test specific effects. Time derivatives were included in the basis functions set. Following smoothness estimation, linear contrasts of parameter estimates were defined to test the specific effects of the four conditions (pleasant cue – grey image, unpleasant cue – grey image, pleasant taste – rinse, unpleasant taste – rinse) with each individual data-set. Voxel values for each contrast resulted in a statistical parametric map of the corresponding t statistic, which was then transformed into the unit normal distribution (SPM z). Movement parameters for each person were added as additional regressors in 1st-level analyses.
Second-level fMRI analyses first examined simple main effects of task with one-sample t-tests for all scans (Table DS2 in the data supplement). These results were thresholded at P=0.05 uncorrected and whole-brain cluster corrected (P<0.05 family-wise error (FWE) for multiple comparisons). To examine the effect of group, independent t-tests were implemented in SPM8 for each of the four conditions separately and all data were reported thresholded at P=0.05 uncorrected and whole-brain cluster corrected (P<0.05 FWE for multiple comparisons). Plots of contrast estimates were extracted using the plots tool in SPM8, and WFU Pick Atlas (http://www.fmri.wfubmc.edu/cms/software) was used to display neural activation, with error bars representing the standard error of the mean. Activation coordinates are listed in the stereotactic space of the MNI ICBM 152 brain (Table 2).
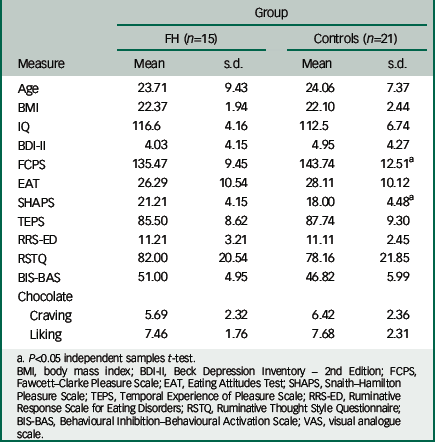
Table 1 Group demographics and psychosocial measures
| Measure | Group | |||
|---|---|---|---|---|
| FH (n=15) | Controls (n=21) | |||
| Mean | s.d. | Mean | s.d. | |
| Age | 23.71 | 9.43 | 24.06 | 7.37 |
| BMI | 22.37 | 1.94 | 22.10 | 2.44 |
| IQ | 116.6 | 4.16 | 112.5 | 6.74 |
| BDI-II | 4.03 | 4.15 | 4.95 | 4.27 |
| FCPS | 135.47 | 9.45 | 143.74 | 12.51 a |
| EAT | 26.29 | 10.54 | 28.11 | 10.12 |
| SHAPS | 21.21 | 4.15 | 18.00 | 4.48 a |
| TEPS | 85.50 | 8.62 | 87.74 | 9.30 |
| RRS-ED | 11.21 | 3.21 | 11.11 | 2.45 |
| RSTQ | 82.00 | 20.54 | 78.16 | 21.85 |
| BIS-BAS | 51.00 | 4.95 | 46.82 | 5.99 |
| Chocolate | ||||
| Craving | 5.69 | 2.32 | 6.42 | 2.36 |
| Liking | 7.46 | 1.76 | 7.68 | 2.31 |
a P<0.05 independent samples t-test.
BMI, body mass index; BDI-II, Beck Depression Inventory – 2nd Edition; FCPS, Fawcett–Clarke Pleasure Scale; EAT, Eating Attitudes Test; SHAPS, Snaith–Hamilton Pleasure Scale; TEPS, Temporal Experience of Pleasure Scale; RRS-ED, Ruminative Response Scale for Eating Disorders; RSTQ, Ruminative Thought Style Questionnaire; BIS-BAS, Behavioural Inhibition–Behavioural Activation Scale; VAS, visual analogue scale.
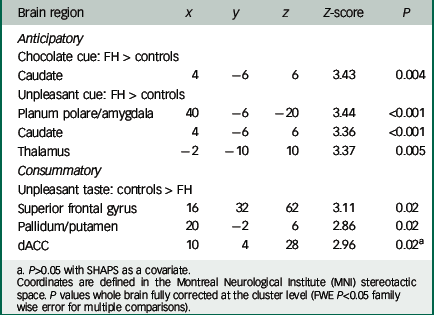
Table 2 Group differences in neural responses to reward and aversion
| Brain region | x | y | z | Z-score | P |
|---|---|---|---|---|---|
| Anticipatory | |||||
| Chocolate cue: FH > controls | |||||
| Caudate | 4 | −6 | 6 | 3.43 | 0.004 |
| Unpleasant cue: FH > controls | |||||
| Planum polare/amygdala | 40 | −6 | −20 | 3.44 | <0.001 |
| Caudate | 4 | −6 | 6 | 3.36 | <0.001 |
| Thalamus | −2 | −10 | 10 | 3.37 | 0.005 |
| Consummatory | |||||
| Unpleasant taste: controls > FH | |||||
| Superior frontal gyrus | 16 | 32 | 62 | 3.11 | 0.02 |
| Pallidum/putamen | 20 | −2 | 6 | 2.86 | 0.02 |
| dACC | 10 | 4 | 28 | 2.96 | 0.02 a |
a P>0.05 with SHAPS as a covariate.
Coordinates are defined in the Montreal Neurological Institute (MNI) stereotactic space. P values whole brain fully corrected at the cluster level (FWE P<0.05 family wise error for multiple comparisons).
Results
Participant characteristics
The two groups were matched for age, BMI and chocolate eating/liking (Table 1). There were significant differences between the control group and the FH group in the measures of anhedonia (Snaith–Hamilton Pleasure Scale, F(31)=1.182, P<0.05, FCPS, F(31)=1.914, P<0.05). There were no significant differences between groups on measures of depression (Beck Depression Inventory-II), attitudes to eating (Eating Attitudes Tests), pleasure (Temporal Experience of Pleasure Scale), motivation (Behavioural Inhibition/Behavioural Activation Scale) or rumination (Ruminative Response Scale for Eating Disorders, Ruminative Thought Style Questionnaire) (Table 1). On the day of the scan, there were no significant differences between groups on the VAS, the BFS and on subjective ratings of hunger (see Table DS1).
Ratings of stimuli
Wanting
A repeated-measures ANOVA with one factor, condition, with two levels (chocolate/aversive), was used to analyse group (FH and controls) wanting of stimuli. As expected, there was a significant main effect of condition on wanting ratings (F(1,70)=1839.260, P<0.001), with all participants wanting the chocolate significantly more than the aversive stimuli (t(71)=40.725, P<0.001). The ANOVA revealed a significant interaction between condition and group on participants’ wanting ratings (F(1,70)=13.298, P=0.001). t-Tests revealed that controls wanted the chocolate significantly more than the FH group (t(70)=2.636, P=0.01); the control group also wanted the aversive stimuli significantly less than the FH group (t(70)=−2.382, P<0.05) (Fig. DS1).
Liking
To analyse the groups’ (FH and controls) liking of the stimuli, a repeated-measures ANOVA with one factor, condition with three levels (chocolate, tasteless rinse and aversive), was used. As expected, there was a significant main effect of condition on all participants’ liking ratings (F(2,68)=172.295, P<0.001) with all participants liking the chocolate significantly more than the rinse (t(106)=11.10, P<0.001) and the aversive stimuli (t(70)=19.528, P<0.001). Despite no significant interaction between condition and group (F(2,68)=2.682, P=0.076), post hoc t-tests revealed that the control group liked the chocolate stimuli significantly more than the FH group (t(34)=2.028, P=0.05) (Fig. DS1). There were no group differences in participants’ liking of tasteless rinse (t(70)=−1.761, P=0.083) or of the aversive stimuli (t(34)=−1.405, P=0.169).
Button presses to complete reward effort phase
Repeated-measures ANOVAs with two factors, difficulty (easy and hard) and group (FH and controls), were used to analyse button presses on the chocolate trials. There was a significant main effect of difficulty on participants’ number of button presses (F(1,34) = 166, P<0.001), with all participants pressing more on hard chocolate trials (Fig. DS2a). There was also a significant interaction between difficulty and group (F(1,34)=14.98, P<0.001), with the controls pressing more than FH for the hard chocolate trials (t(34)=3.449, P=0.002).
Button presses to complete aversive effort phase
Repeated-measures ANOVAs with two factors, difficulty (easy and hard) and group (FH and controls), were used to analyse button presses on the aversive trials. There was a significant main effect of difficulty on participants’ number of button presses (F(1,34)=167, P<0.001), with all participants pressing more on hard chocolate aversive trials (Fig. DS2b). There was also a main effect of group (F(1,34)=10.7, P=0.002) and significant interaction between difficulty and group (F(1,34) =10.6, P=0.003), with the controls pressing more than FH for the hard aversive trials (t(34)=3.275, P=0.002).
Time taken to complete reward effort phase
Repeated-measures ANOVAs with two factors, difficulty (easy and hard) and group (FH and controls) were used to analyse time taken on the chocolate trials. There was a significant main effect of difficulty on participants’ number of button presses (F(1,34)=368, P<0.001), with all participants being faster on chocolate easy trials (Fig. DS3a). There was also a main effect of group (F(1,34)=16.4, P<0.001) and significant interaction between difficulty and group (F(1,34)=14.8, P≤0.001), with the FH taking longer than the controls on the chocolate easy trials (t(34)=−3.98, P<0.001) and hard trials (t(34)=−3.67, P<0.001) (Fig. DS3a).
Time taken to complete aversive effort phase
Repeated-measures ANOVAs with two factors, difficulty (easy and hard) and group (FH and controls), were used to analyse time taken on the aversive trials. There was a significant main effect of difficulty on participants’ number of button presses (F(1,34)=334, P<0.001), with all participants being faster on aversive easy trials (Fig. DS3b). There was also a main effect of group (F(1,34)=10.66, P=0.003) and significant interaction between difficulty and group (F(1,34)=10.38, P=0.003), with the FH taking longer than the controls on the aversive easy trials (t(34)=−3.26, P=0.003) and hard trials (t(34)=−2.75, P=0.009) (Fig. DS3b).
Accuracy
It was expected that easy trials would be successfully completed, and participants would achieve the chocolate taste and on aversive easy trials avoid the aversive taste and instead receive the tasteless rinse. It was also expected that the hard trials would not be completed, and participants would fail to achieve the chocolate and instead receive the tasteless rinse on chocolate trials and fail to avoid the aversive taste on hard aversive trials. Therefore, an error here means that participants did not perform as expected. Using independent samples t-tests, we found that the FH group made more errors for both the chocolate easy trials (t(34)=1.221, P<0.001) and the aversive easy trials (t(34)=10.437, P<0.05) compared with the controls (Fig. DS4).
fMRI response
Main effects of task
Table DS2 provides a summary of the results for each contrast across all participants to indicate the main effect of task. Sights of the food stimuli (anticipation phase) activated regions including the occipital cortex, the ACC and lateral orbitofrontal cortex. As expected, the tastes activated areas such as the insula and ventral striatum (see Table DS2).
Main effects of group
Table 2 provides a summary of the results of the interaction with group (FH v. control). As expected, there were no significant differences in response to the taste of chocolate between the two groups in the primary taste cortex (i.e. anterior insula), confirming that the sensory experience of chocolate was associated with a similar neural response across groups (Table 2). As participants showed significant differences in measures of anhedonia (FCPS and SHAPS), and these measures were shown to be highly correlated: r=−0.492 and P<0.01; SHAPS scores were therefore entered as a covariate into the fMRI analysis. With only two exceptions, the fMRI results remained significant after global SHAPS scores were added as a covariate.
Anticipation
In support of our previous findings with recovered AN, we found increased activation during the anticipation phase (chocolate pictures and aversive pictures) in the FH group compared with the control group in the planum polare/amygdala region (Fig. 2) and the caudate/thalamus region (Fig. DS5) (Table 2).

Fig. 1 Stimuli with jittered inter-trial intervals (layout for a reward − hard trial).
Consummation
However, we found reduced activation in the FH group compared with the control group for the unpleasant taste in the pallidum/putamen region (Fig. 3; Table 2) the superior frontal gyrus (SFG) and the dorsal anterior cingulate cortex (dACC) (Fig. DS6). We found no differences between the groups for the chocolate taste.
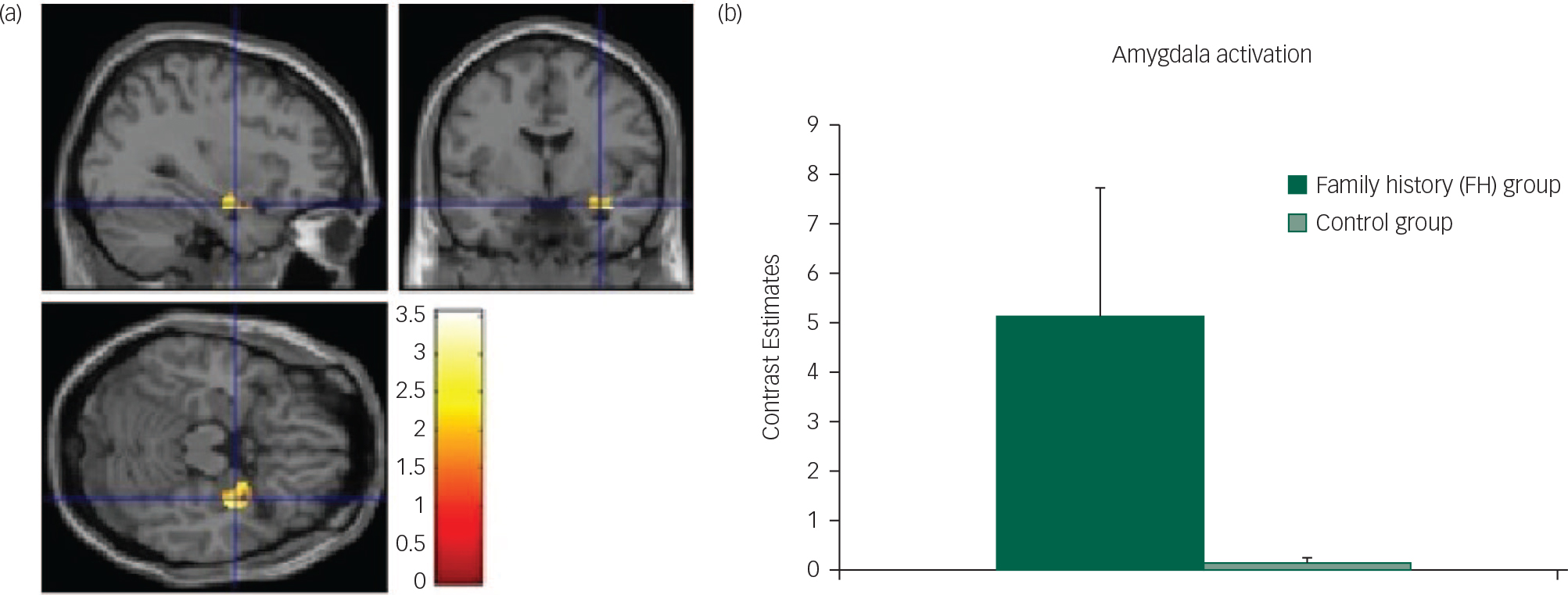
Fig. 2 Unpleasant cue: (a) axial, sagittal and coronal image of increased amygdala activation in FH v. control group; (b) contrast estimates centred at −30, −2, −20 (amygdala) in FH and control groups.
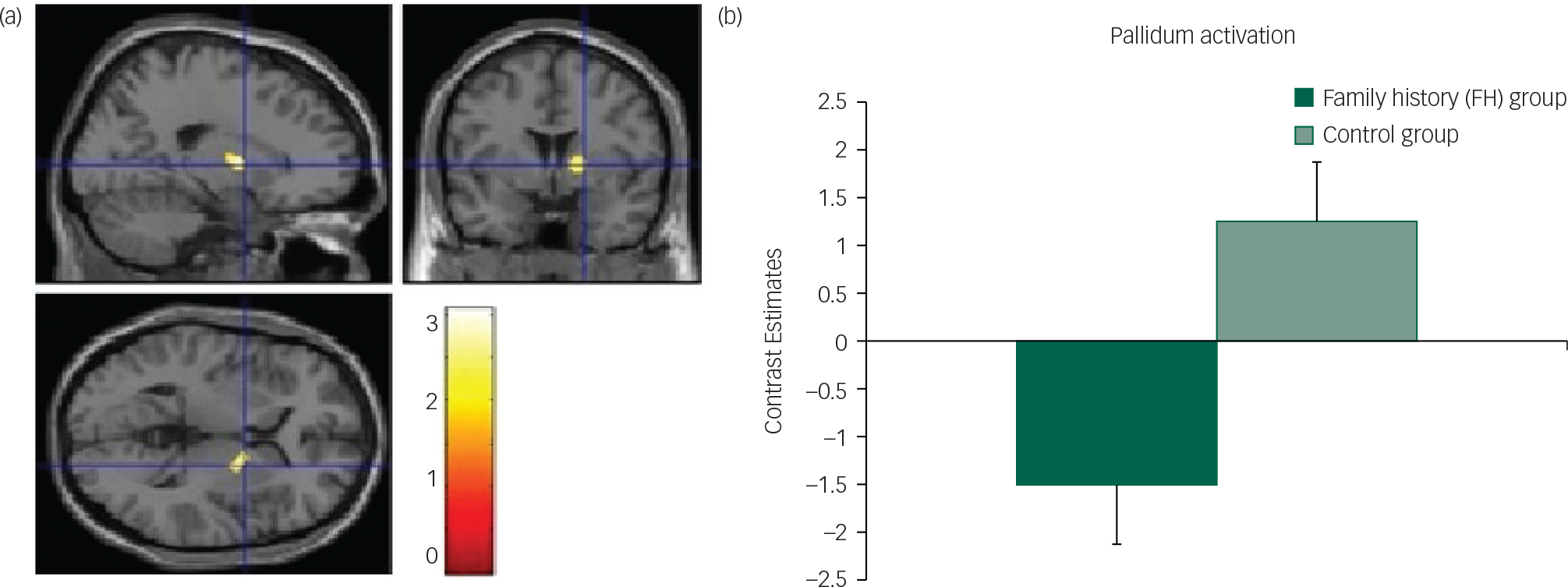
Fig. 3 Unpleasant taste: (a) axial, sagittal and coronal image of decreased pallidum activation in FH v. control group; (b) contrast estimates centred at 20, −2, 6 (pallidum) in FH and control groups.
Discussion
In this study, we examined the neural response during the anticipation and consummation of rewarding and aversive food stimuli in those with a family history of AN, by virtue of having a sister with AN, compared with controls with no family history. We found that there were increased activations to the anticipation of both rewarding and aversive food but decreased responses to the consummation of aversive food.
Specifically, we found that the FH group had increased caudate, thalamus and amygdala activations to the anticipation of both pleasant and unpleasant food. These results are consistent with our previous study which found that females with a personal history of AN also had increased neural responses during the anticipation of food.Reference Cowdrey, Park, Harmer and McCabe 16 As discussed in a recent review by Zhu et al, examining mostly visual food stimuli in AN, increased activation such as this could be related to increased emotional arousal in response to food.Reference Zhu, Hu, Wang, Chen, Guo and Li 2 Brain regions such as the caudate are thought to be involved in valence and salience coding of rewards, and have been found more active in weight-concerned women when choosing high-energy food from a selection of picturesReference van der Laan, de Ridder, Viergever and Smeets 36 and in a meta-analysis of studies on food cue processing in AN patients suggested to be coding the emotion of disgust.Reference Zhu, Hu, Wang, Chen, Guo and Li 2 Therefore, our result of increased activity in these regions during anticipation of food might suggest a mechanism for increased negative emotional reactions in those at risk of eating disorders. In fact, consistent with this notion, the FH group reported wanting the food less after seeing the cue than did the control participants, supporting a negative emotional response during the anticipatory phase; however, further exploratory correlational analysis did not find any relationship in the FH or control group between brain responses in the amygdala and liking or wanting ratings for the aversive taste and cue. This may be due both to the volunteers being healthy and a small sample size.
The increased amygdala response in our study during the anticipation phase of the unpleasant cue in the FH group, compared with the controls, might be due to increased disgust as amygdala activation has been found to correlate with disgust ratings in AN patients when confronted with food stimuliReference Joos, Saum, van Elst, Perlov, Glauche and Hartmann 37 and is known to be activated by aversive stimuli from multiple sensory modalities.Reference Zald 38 Taken together, our results show that even in those ‘at risk’ of AN but with no current symptoms or history of starvation, there are hyperactivations to the anticipation of food, supporting the idea that participants with AN may be highly responsive to food cues, perhaps as a mechanism to predict and control the anxiety produced by food that may be associated with subjective unpleasantness.Reference Kaye, Wierenga, Bailer, Simmons and Bischoff-Grethe 39 , Reference Kaye, Wierenga, Bailer, Simmons, Wagner and Bischoff-Grethe 40
Interestingly, during the consummation phase of the task we found reduced neural responses in the FH group compared with the control group specifically for the unpleasant taste and in the dACC, pallidum/putamen and SFG. The ACC has been implicated in the coding of sensory-hedonic responses to taste and innervates the striatum, where behavioural responses are then computed.Reference Kaye, Wierenga, Bailer, Simmons and Bischoff-Grethe 39 Thus, reduced activity in this region in the FH group might indicate a mechanism by which there is a vulnerability towards reduced liking of food in AN; this was also supported by the behavioural responses of reduced liking for the chocolate in the FH group. Furthermore, as the SFG is involved in self-awareness and rumination in eating disordersReference Goldberg, Harel and Malach 41 , Reference Brooks, O'Daly, Uher, Friederich, Giampietro and Brammer 42 and the pallidum has been identified as a ‘hedonic hotspot’ with a pivotal role for food liking in the brain,Reference Berridge, Robinson and Aldridge 9 it seems that diminished responses in these regions in the FH group could explain a mechanistic predisposition towards reduced pleasure from food tastes.
As no differences between the FH group and controls were found in the activations of the primary taste cortex (anterior insula), our results are unlikely to be related to differences in physical sensitivity to taste and oral texture between the groups. Interestingly, in our previous study in those ‘at risk’ of anorexia, namely recovered AN patients, we found similar regions activated to the aversive taste such as the putamen and dACC. However, unlike the previous study of increased responses in those recovered, we find decreased response in the FH group. Therefore, we suggest that decreased activity in these regions to the unpleasant tastes is more like a trait marker which might only switch to hypersensitivity (increased activation) after experiencing AN (state) and is perhaps modulated by the amount of starvation experienced. Further studies, longitudinal in nature, are of course needed to clarify this.
Taken together our results suggest that the neural response during anticipatory and consummatory responses to rewarding and aversive food stimuli might be a biomarker for risk of developing AN in the future. Therefore, interventions with those at risk of AN that can normalise these differences in brain activity may be helpful in preventing the disorder onset. To clarify this, longitudinal studies investigating the neurobiological profiles of adolescents and young women at risk of AN are needed.
Acknowledgements
We thank Ewelina Rzepa for helping with the scanning.
Funding
This study was supported by the University of Reading start-up fund for C.M.








eLetters
No eLetters have been published for this article.