Muscular ventricular septal defects account for 10–15% of congenital ventricular septal defect,Reference Fu1 the frequency of which is 5.7% in pre-term infants and 1.1–5.3% in term infants.Reference Miyake2 Muscular ventricular septal defects were classified by geographic location as apical, inlet, outlet, and trabecular muscular defects.Reference Lopez, Houyel and Colan3 Currently, transcatheter muscular ventricular septal defects closure is a viable alternative to surgical intervention.Reference Rahmath, Numan and Dilawar4 However, the management of muscular ventricular septal defects often remains a challenge to cardiologists since the defect may be multiple and of varying size and shape. The Cardi-O-Fix plug of Starway Medical Technology Inc. is a permanent implantable device for percutaneous transcatheter closure of abnormal vascular access.Reference Sudhakar, Jose and George5 In this article, we describe our experience for transcatheter closure of muscular ventricular septal defects in five patients using Cardi-O-Fix plug.
Material and methods
Between November 2017 and August 2019, five patients (one male, four females) with muscular ventricular septal defects were selected for transcatheter closure. Two-dimensional echocardiography showed defects in five patients. This study was approved by our local Institutional Review Board. Informed consent was obtained from the parents.
Device
The Cardi-O-Fix plug (Starway Medical Technology Inc., Beijing, China) is constructed from 0.004 in. (0.01 cm) Nitinol wire, with a 5-mm connecting waist approximately as large as the defect to be closed. Polyethylene terephthalate non-woven fabrics are sewn into both retention discs and the waist. Both the left ventricle retention skirt and the right ventricle retention disc are 2 mm larger than the waist. The delivery sheath has a working length of 100 cm, and the leading tip of it is soft and has no an angle (Fig 1).

Figure 1. Cardi-O-Fix plug.
Procedure
The procedures were performed under general anaesthesia with transthoracic echocardiography and fluoroscopic guidance. Heparin (100 U/kg) and prophylactic antibiotics (Cefazolin) were administered intravenously during the procedure. The maximum size of the defect was determined by either transthoracic echocardiography (Fig 2a and b) or left ventriculogram (Fig 3).
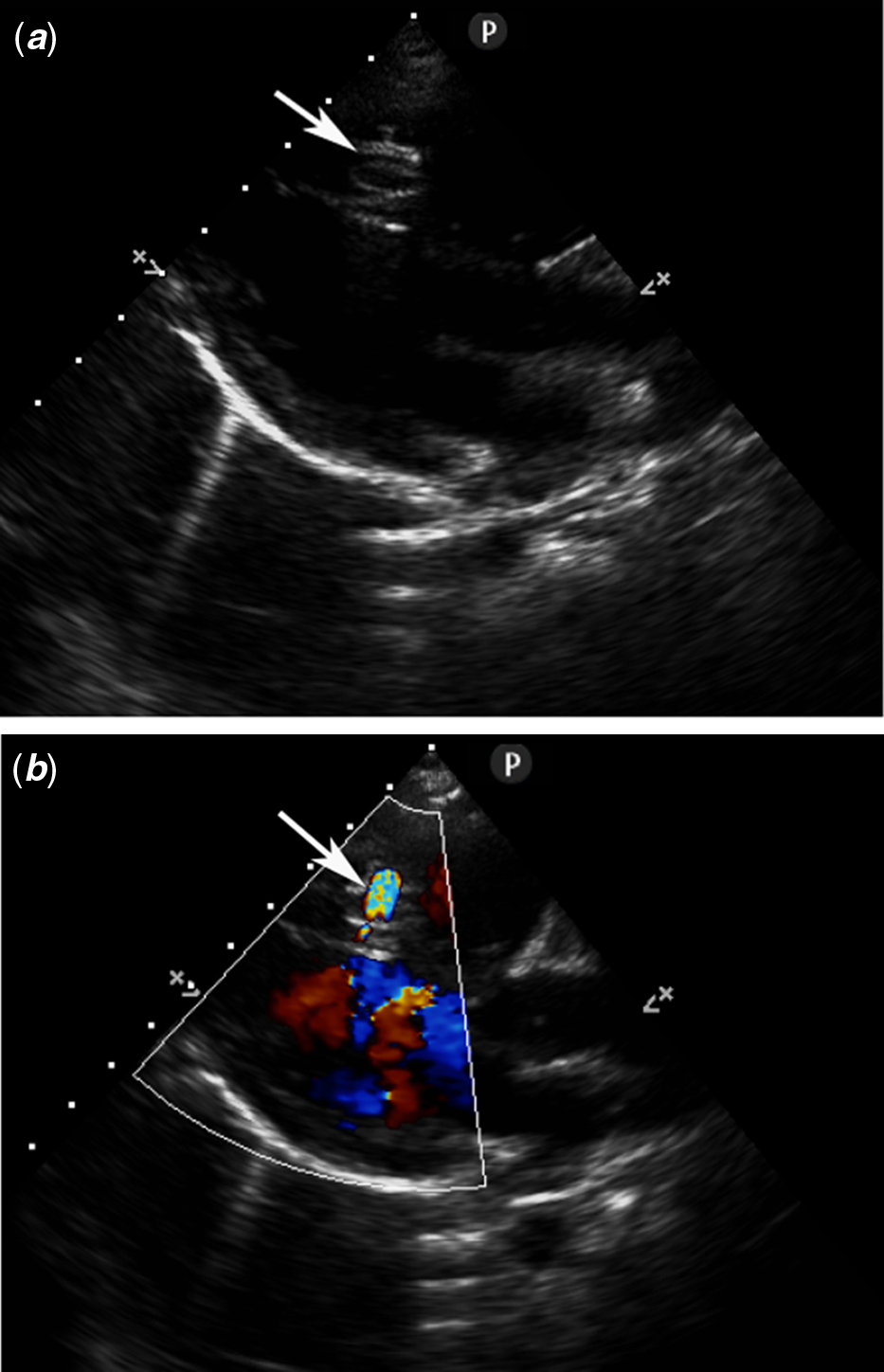
Figure 2. Parasternal long-axis view without (a) and with (b) colour Doppler demonstrating the MVSD (arrow). MVSD, muscular ventricular septal defect.

Figure 3. LV angiogram showing the MVSD (arrow) in RAO view. LV, left ventricle; MVSD, muscular ventricular septal defect; RAO, right anterior oblique.
A Terumo wire was threaded through the femoral artery into the left ventricle and across the defect into the right ventricle using 5 F Judkin’s right coronary catheter. A basket net wire was advanced into the right side of the heart from the femoral vein through the MPA2 right side of the heart catheter. Subsequently, the Terumo wire was snared by the basket net wire in the pulmonary artery or superior vena cava and gently pulled out through the femoral vein (Fig 4). After an arterio-venous guidewire circuit was established, a 5 F delivery sheath (Starway Medical Technology Inc.) was passed over the guide wire from the femoral vein and across the defect into the left ventricle. The wire was then removed. Subsequently, the Cardi-O-Fix plug (10–12 mm) screwed onto the delivery cable was pushed into the delivery sheath and advanced into the left ventricle. The distal disc was gently deployed. The delivery cable was then withdrawn together with the long sheath until resistance is felt and the proximal disc was released. The position of the device and residual shunt was evaluated by the transesophageal echocardiogram and left ventriculogram. Once the position was corrected, the Cardi-O-Fix plug was released by rotating the delivery cable in a counter-clockwise direction (Fig 5a and b).

Figure 4. Anterio-posterior view showing the exchange wire snared in the pulmonary artery (arrow).

Figure 5. Deployment of the RV disc (arrow) (a) and final cine image showing final device position after deployment (b) RV, right ventricle.
Oral aspirin 3 mg/kg daily was recommended for at least 6 months. Electrocardiography, echocardiography, and chest radiography were performed at 24 hours (Fig 6a and b), 6 weeks, 6 months, and 1 year following the procedure.

Figure 6. Four-chamber view without (a) and with (b) colour Doppler showing the closure device (arrow).
Results
The baseline characteristics are shown in Table 1. Transcatheter closure of muscular ventricular septal defects was successful in all five cases after deployment of the device using the Cardi-O-Fix plug (10–12 mm). The size of the left ventricular median defect on left ventricular angiography was 5.6 mm (range: 5.3–7.0). The size of the right ventricular median defect on left ventricular angiography was 3.9 mm (range: 3.3–4.7). The QP/QS ratio was 0.8:1.1. The pulmonary artery pressure was 25–35 mmHg. All the patients underwent successful device implantation with no displacement or detachment. No residual shunt was detected on angiography immediately after the procedure and by colour flow imaging after 24 hours (Fig 3). The cardiac catheterisation data for the five patients are shown in Table 2.
Table 1. Baseline characteristics

Abbreviations: F: female; LV: left ventricle; M: male; MVSDs: muscular ventricular septal defects; RV: right ventricle
Table 2. Catheterisation details
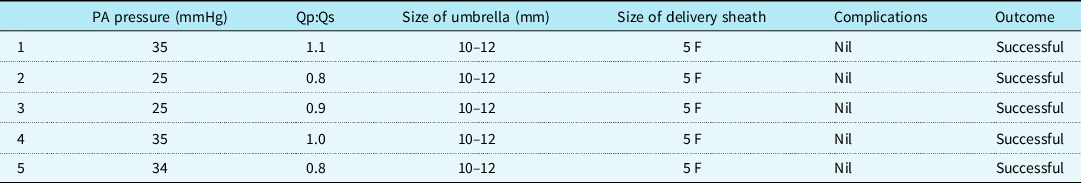
Abbreviations: PA: pulmonary artery; Qp: pulmonary blood flow; Qs: systemic blood flow
Follow-up
Patients have complete echocardiographic closure at the 1-year follow-up. There were no occluder-related arrhythmia, chordae tendineae rupture, tricuspid insufficiency, aortic regurgitation, haemolysis, or embolisation.
Discussion
Muscular ventricular septal defects are often located within the trabecular muscle of the right ventricle,Reference Lopez, Houyel and Colan3 which is difficult for surgical closure resulting from technical difficulties in target localisation. In recent years, percutaneous transcatheter closure was introduced as an alternative to surgery since it has the advantages of minimal trauma in surgery and reliable satisfactory outcome. Transcatheter closure of muscular ventricular septal defects was carried out for the first time by Lock et alReference Lock, Block, McKay, Baim and Keane6 using a Rashkind double umbrella. However, transcatheter closure of muscular ventricular septal defects is still challenging because of the complexity and abnormity of anatomical structures. In an original article published in 2006 by Mulyadi et alReference Djer, Haifa Abdul, Mazeni, Hasri and Kandavello7 where seven patients were catheterised with the Amplatzer muscular ventricular septal defects Occluder, all patients had successful transcatheter closure of muscular ventricular septal defects except one who failed due to the acute angulation course of the defect. In another study, Peter et alReference Peter, Claus, Jan-Christian, Viktor and Martin8 performed transcatheter closure of muscular ventricular septal defects in 17 patients; however, they had to abandon implantation in a small patient because the device did not follow the sharp bending of the delivery sheath. Therefore, an effective and feasible technique and device should be considered carefully by the operator.
The Cardi-O-Fix plug (Starway Medical Technology Inc., Beijing, China) is a cylindrical, self-expandable implantable device made from a nitinol wire mesh. The disc and connecting waist are filled with polyethylene terephthalate non-woven fabric which is sewn securely into the cylinder to increase the thrombogenicity of the device. The utility device is characterised in that the torsion angle of double discs can be adjusted within a certain range, and the connecting waist has a good stretching and bending performance. In this series, the Cardi-O-Fix plug was used to close muscular ventricular septal defects in five patients. We demonstrated that Cardi-O-Fix plug can be effectively utilised to close muscular ventricular septal defects in these children.
With the improvement of devices technology, second generation of Amplatzer occluders has many merits, such as softness, low profile, fabric patch-free, easy to be used in cases of ventricular septal defect. GhoshReference Ghosh, Sridhar, Solomon and Sivaprakasham9 et al used Amplatzer Duct occlude II device for closure of ventricular septal defect in aortic valve prolapse and aortic regurgitation. As in another study, Amplatzer vascular plug II was applied in 12 patients and ADO II in 51 patients for transcatheter perimembranous ventricular septal defects closure.Reference Mijangos-Vázquez, El-Sisi and Sandoval Jones10 HuaReference Hua, Aquino and Owada11 et al successfully completed the transcatheter closure in 16 patients with perimembranous ventricular septal defect by using Amplatzer vascular plug II. These results may be suggested that the new generation of Amplatzer occluders is suitable for the treatment of muscular ventricular septal defect. However, the conclusion still needs to be further verified and confirmed by the clinical application. The purpose of this study was to describe our experience with muscular ventricular septal defect patients who underwent transcatheter closure using the Cardi-O-Fix plug due to its excellent curve-passing performance.
Conclusions
In conclusion, transcatheter closure using Cardi-O-Fix plug is safe and effective in patients with muscular ventricular septal defects. A longer follow-up with a larger number of patients is required to determine the clinical and application values of the procedure in this group of patients.
Acknowledgements
We acknowledge past and present members and our colleagues in the department of Cardiology at the First Affiliated Hospital of Kunming Medical University.
Financial support
This wok was financially supported by the Scientific Research Fund Project of Yunnan Provincial Education Department (2020J0169).
Conflict of interest
None.
Ethical standards
This study was approved by the Institutional Research Ethics Committee of Kunming Medical University and followed the guidelines of the Declaration of Helsinki. All study participants signed informed consent forms.











