Background
During the first wave of the coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), patients with confirmed cases in New York State accounted for roughly 25% of total COVID-19 cases in the USA. Psychiatric hospital in-patients were at particularly high risk for COVID-19 infection, with rates approaching those found in nursing homes.Reference Druss1 Patient populations with serious mental illness may be particularly vulnerable to severe infection because close to half of these patients have one or more comorbidities,2 and in fact those with schizophrenia spectrum disorder have an increased risk of COVID-19 mortality.Reference Nemani, Li, Olfson and Blessing3
Since early 2020 when the pandemic began there have been several observational studies examining the potential benefits of mental health medications, such as antipsychotics and antidepressants, on the course and severity of COVID-19 disease outcomes. Regarding antipsychotics, although theorised to have beneficial effects,Reference Muric, Arsenijevic and Borovcanin4 observational studies of antipsychotic treatment have to date shown no significant benefit on severe outcomes of COVID-19 disease.Reference Hoertel, Sánchez-Rico and Vernet5,Reference Hoertel, Sánchez-Rico and Vernet6 On the other hand, two large observational studies conducted in Europe (France and Spain), both demonstrated the significant, and positive association between antidepressant use and a reduced risk of intubation or death in patients admitted to hospital with COVID-19.Reference Hoertel, Sánchez-Rico and Vernet7,Reference Diez-Quevedo, Iglesias-González and Giralt-López8 This data is strongly supported by the encouraging findings from clinical trials of the selective serotonin reuptake inhibitor (SSRI) fluvoxamine in the USA, in which treated patients exhibited a significantly lower likelihood of clinical deterioration,Reference Lenze, Mattar and Zorumski9 or showed improved respiratory rates and significantly lower incidence of subsequent admission to hospital approximately 2 weeks after the onset of treatment.Reference Seftel and Boulware10
It has also been postulated that some medications may alter the risk of COVID-19 infection following exposure by obstructing SARS-CoV-2 host cell entry, which occurs via the virus binding to the membrane-bound angiotensin-converting enzyme 2 (ACE2) in the nasal passages and lungs. For example, supplementation with vitamin D has been hypothesised to decrease the risk of infection by normalising ACE2 function following exposure,Reference Mahdavi A11 and supporting this hypothesis, two large general population cohort studies, one in the UKReference Ma, Zhou, Heianza and Qi12 and the other in Spain,Reference Oristrell, Oliva and Casado13 documented a small, but protective effect of vitamin D supplementation on COVID-19 positivity. Of particular interest, in light of the positive effects of antidepressants on the course of COVID-19 disease outcomes,Reference Hoertel, Sánchez-Rico and Vernet7,Reference Diez-Quevedo, Iglesias-González and Giralt-López8 data from a recent in vitro study showed that some antidepressants may also prevent SARS-CoV-2 cell entry via inhibition of the acid sphingomyelinase (ASM)/ceramide system,Reference Carpinteiro, Edwards and Hoffmann14 which is likely required to facilitate ACE2 binding of the SARS-CoV-2 virus.Reference Carpinteiro, Gripp and Hoffmann15 Furthermore, there is a substantial body of work documenting the many antiviral properties of antidepressants, in particular SSRIs and it has been theorised that antidepressants may be effective against SARS-CoV-2 infection.Reference Costa, Santos and Branco16,Reference Pashaei17
Aims
Intriguingly, recent population cohort studies investigating medication effects on COVID-19 riskReference Ma, Zhou, Heianza and Qi12,Reference Oristrell, Oliva and Casado13,Reference Reynolds, Adhikari and Pulgarin18 may be confounded by actual rates of viral exposure in the broad geographical communities studied. We propose that risk of infection could also be examined in an in-patient population setting, where the potential for exposure is likely uniform across the facility, and where these mental health medications/supplements are routinely prescribed. In the present study we tested the hypothesis that medication use, such as antidepressants or vitamin D supplementation, modifies the risk of COVID-19 infection in a long-stay, chronic in-patient psychiatry setting.
Method
We conducted a retrospective cohort study of the in-patient population at The Rockland Psychiatric Center (RPC), a large psychiatric facility for adults operated by the New York State Office of Mental Health (OMH). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human patients were approved by the RPC institutional review board, using a waiver of authorisation for informed consent. The study followed the STROBE reporting guidelines for cohort studies.
During the period between June 2 to 31 July 2020, we collected patient data from the OMH online medical records system, across seven wards in entirety (approximately 50% of the RPC in-patient population). Demographic and clinical details were recorded, plus all current medication use (including PRN medications) within the first wave of the pandemic in New York State. COVID-19 infection was determined by a positive polymerase chain reaction diagnostic test or the presence of antibodies following an enzyme-linked immunosorbent assay. Testing was performed at RPC for all patients from the period 24 March 2020 to 31 July 2021. Those who had refused to be tested (n = 5) were excluded from our analysis.
Fisher's exact or Student's t-tests were employed to compare demographic and clinical characteristics of the RPC sample, by COVID-19 infection status. Logistic regression was then employed to model the main effects of medication on the primary outcome of COVID infection (yes/no). Common medications were tested in the following groups; typical neuroleptic use (yes/no), atypical neuroleptic use (yes/no) and chlorpromazine-equivalent (CPZE) (yes/no) daily dose, mood stabiliser (yes/no), antidepressant (yes/no), benzodiazepine (yes/no), anticholinergic (yes/no), antilipidemic (yes/no), antihypertensive (yes/no), antibiotics (yes/no), antiviral (yes/no), steroids (yes/no) and supplement (yes/no). To control the false discovery rate (FDR, < 5%), P-values were subjected to Benjamini–Hochberg adjustment. Following the statistical analysis strategy employed by Nemani et al,Reference Nemani, Li, Olfson and Blessing3 when significant medication main effects were observed (Benjamini–Hochberg P < 0.05), patient-level characteristics considered related to COVID-19 infection were employed as covariates in demographic-adjusted models (age, gender, ethnicity, psychiatric diagnosis, ward and body mass index (BMI)), followed by fully adjusted models with the additional clinical variables of diabetes, hypertension, respiratory illness and heart disease. Current smoking status was not available for most in-patients from online records and was thus not investigated. As a sensitivity analysis, a stepwise selection procedure (criteria set to P < 0.05) was employed, starting with all variables from the fully adjusted model. In exploratory analyses Fisher's exact tests were employed to test for the association between classes of and individual antidepressant and antipsychotic medication use (when the total sample treated was ≥ n = 5) and COVID-19. All analyses were conducted in Stata v14.2 (Stata, College Station TX).
Results
A total of 165 RPC in-patients were included (Table 1), of whom 91 (55%) were positive for COVID-19. Individuals with COVID-19 did not differ by gender, BMI or the presence of hypertension, respiratory illness or diabetes. There was a trend towards significance for COVID-19 positively to be associated with schizophrenia spectrum disorders (schizophrenia or schizoaffective disorder) when compared with mood disorders (bipolar disorder or major depressive disorder) (P = 0.062), supporting data from a recent study.Reference Nemani, Li, Olfson and Blessing3 There was no difference in COVID-19 infection status when patient diagnoses were further classified into manic and depressive types (see Supplementary Tables 1 and 2 available at https://doi.org/10.1192/bjo.2021.1053).
Table 1 Demographic and clinical characteristics of the RPC sample (n = 165)
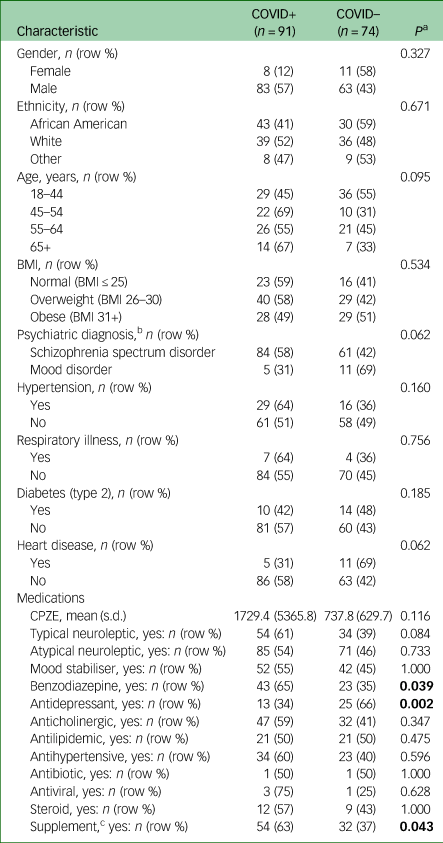
BMI, body mass index; CPZE, chlorpromazine-equivalent daily dose. Significant differences bolded.
a. Fisher's exact or Student's t-test.
b. n = 161 (four participants with unspecified psychiatric diagnoses were excluded).
c. Vitamins B (B complex, B12, folic acid), C, D, multivitamin, fish oil, Calcium carbonate, magnesium, probiotic.
We observed a significant association of CPZE dose with increasing COVID-19 risk (Table 2, odds ratio (OR) = 1.0007, 95% CI 1.0002–1.0013, Benjamini–Hochberg adjusted P < 0.05), which was retained after adjustment for demographic and clinical variables. Interpreting CPZE quartile data from the adjusted model, the probability of being COVID-19 positive increased from 0.41 at the 25th percentile, to 0.44 at the 50th, 0.52 at the 75th, to 0.63 at the 90th percentile of the CPZE daily dose data. The use of benzodiazepines and supplements were also associated with COVID-19 positivity but did not remain significant following Benjamini–Hochberg adjustment.
Table 2 Odds ratios with 95% CIs of the unadjusted and adjusted medication models of COVID-19 infection
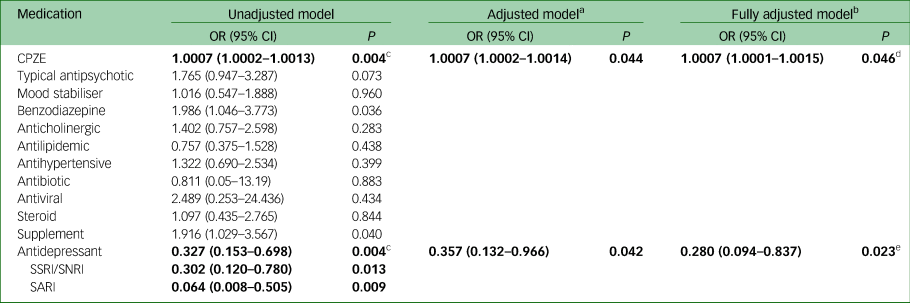
Significant models are in bold. CPZE, chlorpromazine-equivalent daily dose; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin-norepinephrine reuptake inhibitor; SARI, serotonin-2 antagonist reuptake inhibitor.
a. Adjusted for age (categorical: 18–44 years (reference group), 45–54, 55–64, 65+); gender; ethnicity (categorical: African American (reference group), White, Other); psychiatric diagnosis; ward (categorical: 7 levels), BMI (ordinal: normal, overweight, obese).
b. Adjusted as for footnote a, plus for the presence of diabetes, hypertension, respiratory illness or heart disease.
c. Benjamini–Hochberg adjusted P < 0.05
d. Stepwise regression model OR = 1.0007 (1.000004–1.0014), P = 0.049.
e. Stepwise regression model OR = 0.292 (0.102–0.833), P = 0.021.
There was a significant association with antidepressant use (OR = 0.33, 95% CI 0.15–0.70, Benjamini–Hochberg adjusted P < 0.05); those treated with antidepressants had significantly reduced odds of COVID-19 infection, even after full adjustment for all demographic and clinical variables (fully adjusted OR = 0.28, Table 2). Results from sensitivity analyses using a stepwise selection of model variables yielded similar results. Analysis of individual antidepressant classes showed that patients treated with serotonin reuptake inhibitors (SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs)) or serotonin-2 antagonist reuptake inhibitors (SARI), both had a significantly decreased likelihood of COVID-19 infection (Table 2: unadjusted OR = 0.32, 95% CI 0.12–0.78 and OR = 0.06, 95% CI 0.008–0.51, respectively). Exploratory analyses of individual antidepressant use demonstrated a significant association between lower risk of infection and the SSRI antidepressant fluoxetine (P = 0.023), as well as the SARI antidepressant trazodone (P = 0.001), see Supplementary Table 3.
Discussion
Main findings
In this observational study of a long-stay hospital, psychiatric in-patient cohort, we found that patients who received antidepressant medication had a 72% lower odds of testing positive for COVID-19, compared with those not treated with antidepressants. The serotonin reuptake inhibitor (SSRI and SNRI) and the SARI classes of antidepressants appeared to drive the protective effect. Our finding augments recent studies documenting the beneficial effect of antidepressants (including both SSRIs and non-SSRIs) on reducing the risk of intubation or death in patients admitted to hospital with COVID-19,Reference Hoertel, Sánchez-Rico and Vernet7,Reference Diez-Quevedo, Iglesias-González and Giralt-López8,Reference Hoertel, Sánchez-Rico and Gulbins19 and taken together provides support for a randomised controlled trial to test the use of these antidepressants in the management of COVID-19 risk in psychiatric in-patient settings.
Interpretation of our findings
There are a number of plausible pathophysiological mechanisms that could explain the protective effects of antidepressant medication against COVID-19 infection. First, antidepressant use may directly impede viral host cell entry via inhibition of the ASM/ceramide system (Reference Törnquist, Asghar, Srinivasan, Korhonen and Lindholm20,Reference Hoertel, Sánchez-Rico, Cougoule, Gulbins, Kornhuber and Carpinteiro21 and references therein). Specifically, the ASM enzyme, present in lysosomes and the cell membrane, cleaves ceramide from sphingomyelin, resulting in the formation of ceramide-enriched membrane domains in the outer cell membrane. Preclinical studies have suggested that SARS-CoV-2 infection requires activation of the ASM/ceramide system,Reference Carpinteiro, Edwards and Hoffmann14 with viral entry into host cells facilitated by the clustering of ACE2 into these ceramide-enriched membrane domains.Reference Carpinteiro, Gripp and Hoffmann15 Of relevance to our study finding, several antidepressants including the SSRI fluoxetine functionally inhibit ASM activity,Reference Carpinteiro, Edwards and Hoffmann14 and pharmacological in vitro studies have shown that treatment with a number of both SSRI, tricyclic and tetracyclic antidepressants directly block uptake of SARS-CoV-2 by epithelial cells,Reference Carpinteiro, Edwards and Hoffmann14 and with regards to fluoxetine,Reference Zimniak, Kirschner and Hilpert22,Reference Dechaumes, Nekoua, Belouzard, Sane, Engelmann and Dubuisson23 also dramatically reduced viral titers.Reference Schloer, Brunotte, Mecate-Zambrano, Zheng, Tang and Ludwig24
Studies of both cell culture systems and in vivo models have also highlighted the antiviral activity of particular antidepressants. For example, fluoxetine is a potent inhibitor of enterovirus replication,Reference Zuo, Quinn, Kye, Cooper, Damoiseaux and Krogstad25,Reference Bauer, Manganaro and Zonsics26 the SSRI sertraline can inhibit Ebola virus cell entry both in vitro and in vivo, Reference Johansen, DeWald and Shoemaker27 the SSRI citalopram inhibits HIV cell entry and replication,Reference Greeson, Gettes and Spitsin28 and citalopram and sertraline may reduce HIV replication in patient cerebrospinal fluid.Reference Letendre, Marquie-Beck and Ellis29 Antidepressants can also act as anti-inflammatory agents, reducing levels of proinflammatory cytokines.Reference Pashaei17 For example, binding of fluoxetine to the sigma-1 receptor in the endoplasmic reticulum was shown to decrease cytokine activity and enhance survival in preclinical models of sepsis and inflammation,Reference Rosen, Seki, Fernández-Castañeda, Beiter, Eccles and Woodfolk30 and human studies have supported the concept of a general decrease of interleukin (IL)-1β and IL-6 in serum from patients taking antidepressants.Reference Hannestad, DellaGioia and Bloch31 Taken together these studies provide underlying mechanisms for the positive effect of antidepressant use on COVID-19 risk following potential exposure.
Typical antipsychotics have also been theorised to treat COVID-19 symptoms,Reference Muric, Arsenijevic and Borovcanin4 but so far observational studies of antipsychotic treatment (chlorpromazineReference Hoertel, Sánchez-Rico and Vernet5 or haloperidolReference Hoertel, Sánchez-Rico and Vernet6) have not demonstrated a beneficial effect of antipsychotic treatment on COVID-19 disease outcomes and overall mortality. In fact, one large cohort study of adult in-patients with a diagnosis of COVID-19 reported that antipsychotic treatment during COVID admission was significantly associated with a higher mortality rate.Reference Diez-Quevedo, Iglesias-González and Giralt-López8 In our study, we observed a small, but significant association of increasing CPZE daily dose with increased COVID-19 infection, suggesting that use of some antipsychotics may also increase the risk of infection.
Consistent with a prior patient cohort study,Reference Reynolds, Adhikari and Pulgarin18 we did not find an association between use of antihypertensive medication and COVID-19. Conversely, our findings do not support previous population cohort studies documenting a protective effect of vitamin D supplementation on COVID-19 risk.Reference Ma, Zhou, Heianza and Qi12,Reference Oristrell, Oliva and Casado13 Intriguingly, Meltzer et al, recently reported data collected from a large urban academic medical centre, showing an increased risk for testing positive for COVID-19 in patients with a likely vitamin D deficiency, compared with those with sufficient levels.Reference Meltzer, Best, Zhang, Vokes, Arora and Solway32 We and others have reported that vitamin D deficiency is frequent in psychiatric in-patient populations.Reference Clelland, Read and Drouet33 Thus, an explanation for our finding of significantly more patients treated with supplements (including vitamin D) who were COVID-19 positive, may be because of underlying deficiencies that negatively have an impact on COVID-19 risk in this long-stay psychiatric facility.
Limitations
The main limitation of this study was the small size of the in-patient sample investigated. Additionally, severity outcomes following infection were not analysed, primarily because most patients with severe illness were transferred to local hospitals with limited ongoing status reporting in OMH records. Additionally, one of the main hypotheses of this study was that the potential for exposure to COVID-19 was uniform across the RPC facility. Although group programmes at RPC were discontinued in late March 2020, prior to this period we were not able to collect information on daily activities and interactions that could influence risk, which is a limitation of the retrospective chart review protocol. Of relevance to this point, we did not find a significant difference in COVID-19 infection status between patients with a current depressive episode, current mania or psychosis, suggesting that in this study there was not a significant impact on COVID-19 infection status by individual psychopathology.
Implications
A follow-on large cohort study that also evaluates characteristics of COVID-19 infection would be beneficial to confirm these initial but interesting findings, with the ultimate aim of developing medication-based COVID-19 prevention strategies for psychiatric in-patient settings.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2021.1053
Data availability
The data that support the findings of this study are available from the corresponding author (J.D.C.) upon reasonable request and approval by the Nathan S. Kline Institute institutional review board.
Acknowledgments
We thank Dr Fabien Tremeau for his useful and informative discussions on this study.
Author contributions
Concept and design of the study was contributed to by C.L.C. and J.D.C. All authors contributed to the acquisition, analysis and interpretation of data. Statistical analysis was undertaken by C.L.C. Drafting of the initial manuscript was undertaken by C.L.C. and J.D.C. All authors contributed to the critical revision and review of the final manuscript.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of interest
None.






eLetters
No eLetters have been published for this article.