The human gut is home to trillions of microorganisms. These microbes, collectively known as ‘gut microbiota’, are composed of eukarya (0·01 %), protozoa (0·01 %), fungi (0·1 %), archaea (0·1 %), viruses (6 %) and bacteria (93 %)(Reference Thursby and Juge1–Reference Sender, Fuchs and Milo3). The gut microbiota is vastly diverse, where the number of microbes is 10 times higher than germline and somatic cells of the human body(Reference Qin, Li and Raes2,Reference Rinninella, Cintoni and Raoul4–Reference Linares, Ross and Stanton6) . Further, the number of microbial genes (gut microbiome) is over a hundredfold more than the human genes(Reference Qin, Li and Raes2,Reference Rinninella, Cintoni and Raoul4,Reference Adak and Khan5) . It has also been estimated that a normal healthy gut harbours over 1000 microbial species, with at least 160 microbial species shared among individuals(Reference Qin, Li and Raes2,Reference Linares, Ross and Stanton6) .
The metabolic interactions between the gut microbiota and host involve complex interactive processes(Reference Chen, Wang and Kuo7). The gut microbiota undertakes anaerobic fermentation activities to produce a wide range of metabolites, including short-chain fatty acids (SCFA), branched-chain fatty acids, neurotransmitters and others(Reference Blaak, Canfora and Theis8,Reference Williams, Grant and Gidley9) . Each metabolite is an information messenger between microbes and host cells that can affect these interactions(Reference Blaak, Canfora and Theis8,Reference Williams, Grant and Gidley9) .
Furthermore, diet, particularly dietary fibre (DF) intake, is a modifiable lifestyle factor associated with the modulation of the gut microbiota composition and function(Reference David, Maurice and Carmody10–Reference Wu, Compher and Chen12). Intake of fermentable DF may diversify the gut microbiota community, subsequently increasing the production of SCFA(Reference Power, O’Toole and Stanton13). SCFA are considered neuroactive bacterial metabolites of DF degradation and fermentation(Reference Cryan and Dinan14) and also regulate systemic inflammation and oxidative stress in the gut(Reference Swann, Kilpatrick and Breslin15). Several reviews show that SCFA can affect other neuroactive metabolites(Reference Silva, Bernardi and Frozza16), including serotonin(Reference Oleskin and Shenderov17). Therefore, the interactions between DF intake and the gut microbiota are likely crucial for the psychological well-being of the human host(Reference Berk, Williams and Jacka18).
The current understanding of how different types of DF affect the human body has been gleaned from studies on prebiotics, whole food or isolated fibre interventions(Reference David, Maurice and Carmody10). While several reviews have established the protective role of DF against chronic diseases and gut dysbiosis(Reference Reynolds, Mann and Cummings19–Reference O’Grady, O’Connor and Shanahan22), critical gaps remain. Most studies focus on broad DF categories rather than the distinct effects of specific fibre types on gut microbiota composition, function and metabolite production. Moreover, the interconnection among DF, the gut microbiome and psychological well-being remains largely underexplored. Given that microbial and metabolic responses to DF are dictated by its chemical structure(Reference Martínez, Kim and Duffy23,Reference Vinelli, Biscotti and Martini24) , this review addresses the following key questions: (1) How do different DF types impact gut microbiota composition, metabolic activity and psychological well-being? (2) What gaps remain in the current understanding and what future research directions are needed to further investigate these relationships? By addressing these questions, this review aims to provide a more nuanced understanding of how specific fibre types influence the gut microbiome and psychological well-being in adults.
Gut microbiota composition
Gut microbes are classified according to their taxonomy: phyla, classes, orders, families, genera and species(Reference Barlow, Leite and Romano25). Gut microbial density increases progressively downstream from the stomach and duodenum (ranging from 101 to 103 cells per gram) to the large intestine (1011 to 1012 cells per gram)(Reference Schippa and Conte26). The gut microbiota differs across different regions of the intestine due to variations in physiology, pH, oxygen levels and digesta flow rates, substrate availability and host secretions(Reference Flint, Scott and Louis27). The small intestine has relatively short transit times (three to five hours) and high bile concentrations. In contrast, the large intestine has slow flow rates, a neutral to mildly acidic pH and a higher concentration of obligate anaerobic microbes(Reference Rinninella, Cintoni and Raoul4,Reference Adak and Khan5,Reference Flint, Scott and Louis27–Reference Seekatz, Schnizlein and Koenigsknecht29) . The two most abundant gut microbial phyla, which represent 90 % of the gut microbiota, belong to Bacillota (formerly Firmicutes) (Lactobacillus, Bacillus, Enterococcus, Ruminococcus and Clostridium genera) and Bacteroidota (formerly Bacteroidetes) (Bacteroides and Prevotella genera)(Reference Rinninella, Cintoni and Raoul4,Reference Adak and Khan5,Reference Methe, Nelson and Pop28,Reference Seekatz, Schnizlein and Koenigsknecht29) . The phylum Actinomycetota (formerly Actinobacteria) is less abundant in the overall proportion of the gut microbiota but mainly consists of the Bifidobacterium genus(Reference Rinninella, Cintoni and Raoul4,Reference Adak and Khan5,Reference Methe, Nelson and Pop28,Reference Seekatz, Schnizlein and Koenigsknecht29) (Figure 1). For this manuscript, former taxonomic names, Firmicutes, Bacteroidetes and Actinobacteria will be used.
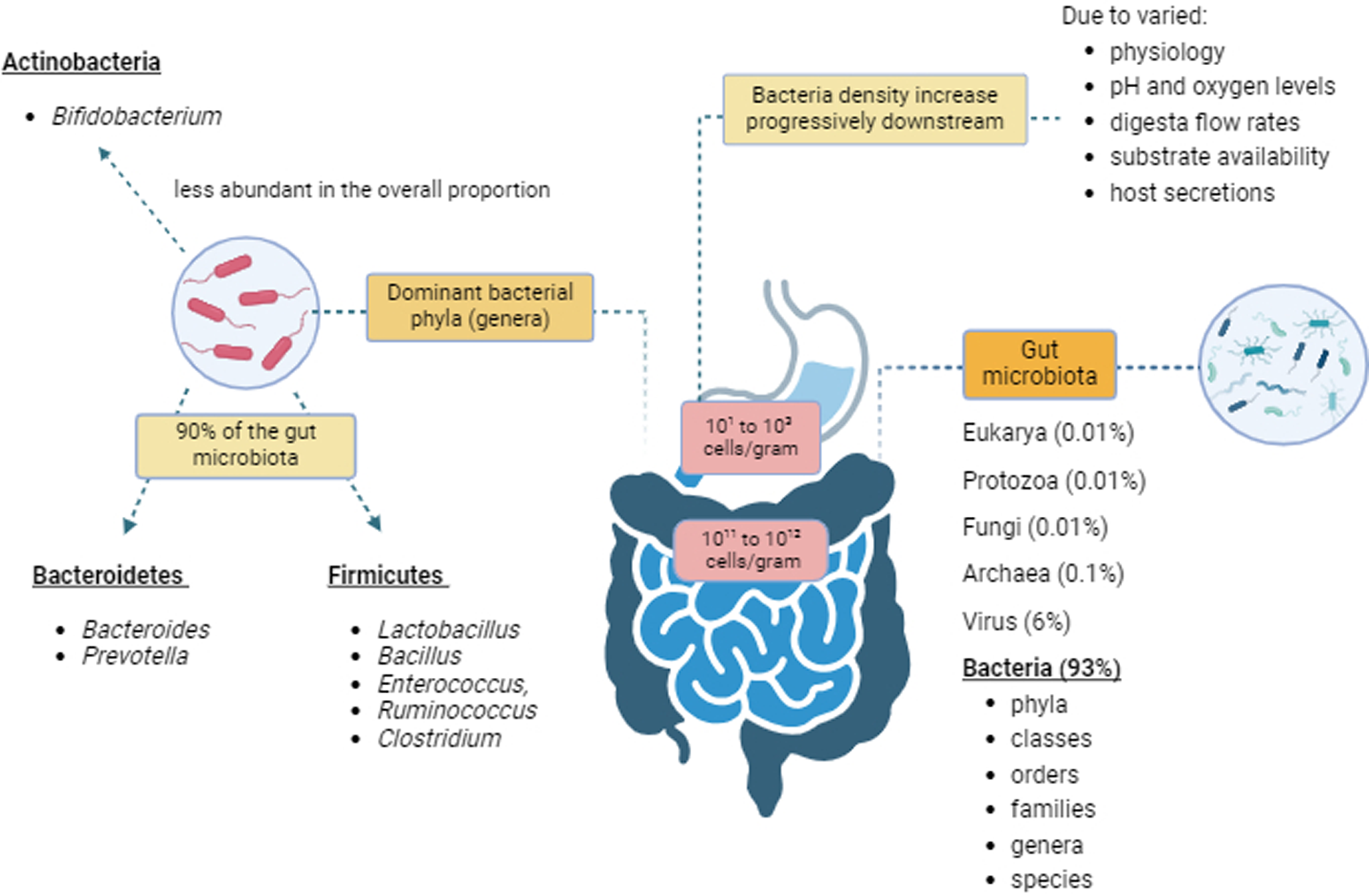
Figure 1. Gut microbiota composition. Created with BioRender.com.
What is a healthy gut microbiota
Due to the high degree of inter-individual variability(Reference Schloissnig, Arumugam and Kota30), there is no consensus on the definition of a ‘healthy gut microbiota’. Nonetheless, it can be described as a state in which the gut microbiota exhibits diversity, stability and resilience to potential gut disturbances(Reference Williams, Grant and Gidley9,Reference Rogowski, Briggs and Mortimer31) or achieves a state of homeostasis(Reference DeGruttola, Low and Mizoguchi32).
Gut homeostasis is a complex interconnection involving the gut microbiota, specialised epithelial cells and the host immune system(Reference Stolfi, Maresca and Monteleone33). These specialised epithelial cells are part of the gut barrier and regulate cross-communication between commensal microbial communities and mucosal immune cells(Reference Peterson and Artis34). Consequently, this helps protect the gut against pathogens(Reference Peterson and Artis34). The state of homeostasis occurs when there is neither an overgrowth of pathogenic microbes nor a loss of beneficial gut microbes and diversity(Reference DeGruttola, Low and Mizoguchi32). The most well-known diversity indices are alpha (richness and evenness of gut microbial species within an individual) and beta diversity (similarity and differences of microbial community between individuals)(Reference Wagner, Grunwald and Zerbe35–Reference Samuthpongtorn, Nopsopon and Pongpirul37). Nourishing the gut microbiota by consuming a diverse diet containing substrates such as DF may help enhance gut microbiota diversity, stability and resilience(Reference Williams, Grant and Gidley9,Reference Rogowski, Briggs and Mortimer31) and/or homeostasis(Reference DeGruttola, Low and Mizoguchi32), promoting a healthier gut microbiota.
Dysbiosis, on the other hand, occurs when gut homeostasis is disrupted(Reference DeGruttola, Low and Mizoguchi32). A dysbiotic microbiota is often related to a loss of microbial diversity(Reference Ling, Liu and Cheng38), which can compromise the functional resilience of the gut microbiota. This subsequently will increase susceptibility to dysbiosis-related diseases, including irritable bowel syndrome (IBS)(Reference Stolfi, Maresca and Monteleone33) and IBS-associated psychological issues, including anxiety and depression(Reference Zamani, Alizadeh-Tabari and Zamani39). Emerging evidence suggests that disruptions in microbial functions, called the ‘functional core’, may be more critical than microbial composition changes in establishing a healthy gut microbiota and psychological health(Reference Redondo-Useros, Nova and González-Zancada40,Reference Bäckhed, Fraser and Ringel41) .
Determining microbial functions is achieved using -omic approaches(Reference Naska, Lagiou and Lagiou42). For instance, metagenomics characterises the various gut microbial species and their gene abundances depending on the depth of sequencing(Reference Chen, Wang and Kuo7); metatranscriptomics allows the comparison of the microbial gene expression profile among individuals(Reference Naska, Lagiou and Lagiou42,Reference Corella and Ordovás43) ; metabolomics and proteomics provide insights into the functional relationship between the gut microbiota and host(Reference Chen, Wang and Kuo7,Reference Corella and Ordovás43) using mass spectrometry (MS) instruments(Reference Chen, Wang and Kuo7,Reference Agus, Clément and Sokol44) . These -omic approaches help improve our understanding of the complexity of the gut microbiota and its role in human health and diseases.
Definition of dietary fibre
Diet, particularly DF intake, is a modifiable lifestyle factor associated with modulating the gut microbiota composition and function(Reference David, Maurice and Carmody10–Reference Wu, Compher and Chen12). The ability of the gut microbes to metabolise DF depends on the chemical structure, including chain length and branching of DF(Reference Holscher45).
Defining DF has been controversial for multiple reasons. Firstly, DF are a collection of related chemical compounds(Reference Jones46). The physicochemical mechanisms that provide health benefits are not fully elucidated. Secondly, whether and how DF affects peristaltic gut movement and microbial fermentation is unclear(Reference Augustin, Aas and Astrup47). Thirdly, compounds embedded in the DF matrix, such as antioxidants and polyphenols, may produce different physical, chemical and physiological effects in the gut(Reference Surampudi, Enkhmaa and Anuurad48). It is also unclear if these effects are contributed by DF per se or occur only within the food matrix(Reference Jones46). In a food industry context, DF are further characterised based on physiological effects(Reference Jones46,Reference Williams, Mikkelsen and Flanagan49) . These complexities around defining DF have led to separate organisations proposing their own definitions.
Despite DF complexities, a uniform and accurate definition is warranted. In 2009, the CODEX Alimentarius Commission proposed a definition and classified DF into three distinct categories(Reference Howlett, Betteridge and Champ50). The first category covers DF derived from naturally occurring foods as part of a healthy diet. The second and third categories include extracted DF and synthetic carbohydrate polymers, which have demonstrated beneficial health effects(Reference Howlett, Betteridge and Champ50). These effects may include increased gut transit time and faecal bulk, colonic fermentation and modulation of blood glucose and cholesterol levels(Reference Jones46). The CODEX definition allows officials worldwide to decide whether to include oligosaccharides and/or carbohydrates of three to nine monomeric units within the DF definition(Reference Howlett, Betteridge and Champ50,Reference Stephen, Champ and Cloran51) . While no uniform definition is used worldwide, CODEX definition adaptation could be an initial step towards achieving global DF definition consensus.
Prebiotics
Most prebiotics are DF, but not all DF are prebiotics(Reference Holscher45). Prebiotics are usually carbohydrates, and only a few functional carbohydrates have been accepted as prebiotics. These include inulin-type fructans, fructo-oligosaccharides (FOS)(Reference Gill, Rossi and Bajka20,Reference Verspreet, Damen and Broekaert52,Reference Gibson, Hutkins and Sanders53) and galacto-oligosaccharides (GOS)(Reference Dewulf, Cani and Claus54,Reference Vandeputte, Falony and Vieira-Silva55) that effectively stimulate the growth of species from the Bifidobacterium (Reference Shoaib, Shehzad and Omar56,Reference Moens, Verce and De Vuyst57) and Lactobacillus genera(Reference Zeng, Chen and Chen58). Resistant starch (RS)(Reference Gong, Cao and Chi59,Reference Broekaert, Courtin and Verbeke60) and arabinoxylans(Reference Gong, Cao and Chi59,Reference Slavin61,Reference Fuller, Beck and Salman62) also have prebiotic effects(Reference Gong, Cao and Chi59,Reference Slavin61,Reference Fuller, Beck and Salman62) . However, most experimental methods and mechanisms of RS and arabinoxylans have been investigated either in vitro (Reference Vardakou, Palop and Christakopoulos63) or in animal models, which require further exploration to confirm their role as a prebiotic for improving human health.
The current concept of prebiotics has been criticised as ill-defined and in need of revision(Reference Deehan, Duar and Armet64). Initially, prebiotics were described as ‘selective’ and ‘specific’ toward beneficial gut microbial groups, which shifts the gut microbiome to a ‘healthier’ state(Reference Deehan, Duar and Armet64). However, this concept lacks clarity, as many dietary compounds could meet these criteria. Moreover, gut microbial species can share degradation features via horizontal gene transfer, allowing a broad range of species with the necessary degrading enzymes to degrade DF(Reference Kaoutari, Armougom and Gordon65). Compounds such as polyphenols and polyunsaturated fatty acids with evident therapeutic effects may also qualify as prebiotics(Reference Gibson, Hutkins and Sanders53). This further challenges the notion of selectivity and identification of beneficial microbes within the definition(Reference Deehan, Duar and Armet64).
Categorising gut microbes as beneficial or non-beneficial may be oversimplified(Reference Deehan, Duar and Armet64). Different gut microbes can be both beneficial and detrimental to the human host depending on environmental factors such as diet, gut microbiota or host genetic predisposition(Reference Deehan, Duar and Armet64). Prebiotic research often focused on bifidobacteria and lactobacilli, as these genera are widely known for their beneficial role(Reference Deehan, Duar and Armet64). However, other genera, such as Faecalibacterium (Reference Dewulf, Cani and Claus54), Anaerostipes and Bilophila (Reference Vandeputte, Falony and Vieira-Silva55), as well as the previously considered harmful genera Clostridia and Bacteroide (Reference Atarashi, Tanoue and Oshima66,Reference Round, Lee and Li67) , may also be beneficial to the human host.
Microbiota accessible carbohydrates
The inter-individual variation of gut microbiota makes defining DF and prebiotics more complex. Gut microbiota composition varies between individuals and populations of different physical and health statuses and lifestyles(Reference Gong, Cao and Chi59). These may impact the degree of metabolism and health effects of DF on the human body. A new term, microbiota-accessible carbohydrates (MAC), was coined to address these challenges. This term classifies carbohydrates into dietary (prebiotics and DF) and host-derived MAC (mucosal glycans)(Reference Sonnenburg Erica and Sonnenburg Justin68). MAC does not include non-fermentable DF and depends on the presence of gut microbial species in the gut to metabolise the different types of DF(Reference Hehemann, Correc and Barbeyron69,Reference Ze, Duncan and Louis70) . For example, individuals who possess the important gut microbial species Rumonococcus bromii can metabolise Resistant Starch (RS) type 3(Reference Ze, Duncan and Louis70). Therefore, RS type 3 would be considered a MAC for these individuals(Reference Ze, Duncan and Louis70). Additionally, if a MAC provides health benefits to the human host, it would also be a prebiotic(Reference Bindels, Delzenne and Cani71). This concept helps contextualise how different DF types interact with the gut microbiota, setting the stage for a deeper discussion on their metabolic activities and implications for host health.
Metabolic activities of the gut microbiota
This section describes how DF metabolism can influence the relationship between the gut microbiota and the human host. The effectiveness of the existing DF interventions on the gut microbiota and psychological well-being is subsequently reviewed.
DF are usually favoured over other nutrients as a substrate for microbial anaerobic fermentation(Reference Blaak, Canfora and Theis8,Reference Power, O’Toole and Stanton13) . Gut microbes can undertake two types of fermentation: saccharolytic and proteolytic. Saccharolytic fermentation mainly occurs in the proximal colon(Reference Power, O’Toole and Stanton13,Reference Schaafsma and Slavin72) . This part of the colon is more acidic (pH 5·5–6·5) than the distal colon (pH 6·5–7·0)(Reference Simpson and Campbell73) and has a greater availability of highly fermentable DF, such as inulin, which produces SCFA(Reference Power, O’Toole and Stanton13,Reference Schaafsma and Slavin72) . Saccharolytic fermentation increases faecal biomass, bulk, weight and frequency(Reference Power, O’Toole and Stanton13,Reference Schaafsma and Slavin72) and are considered beneficial to a certain extent(Reference Blaak, Canfora and Theis8). Proteolytic fermentation, on the other hand, occurs in the distal colon, where there is are lower amount of fermentable DF(Reference Power, O’Toole and Stanton13,Reference Guarner and Malagelada74) . In this process, the high amount of undigested dietary protein is broken down by proteolytic bacteria and subsequently used in proteolytic fermentation(Reference Power, O’Toole and Stanton13,Reference Guarner and Malagelada74) . This fermentation process potentially results in toxic compounds, such as branched-chain fatty acids, ammonia, amines, phenols, thiols and indoles(Reference Power, O’Toole and Stanton13,Reference Guarner and Malagelada74) (Figure 2). Therefore, consuming a wide range of DF will likely benefit the human host as it promotes saccharolytic fermentation and lowers proteolytic fermentation(Reference Schaafsma and Slavin72).
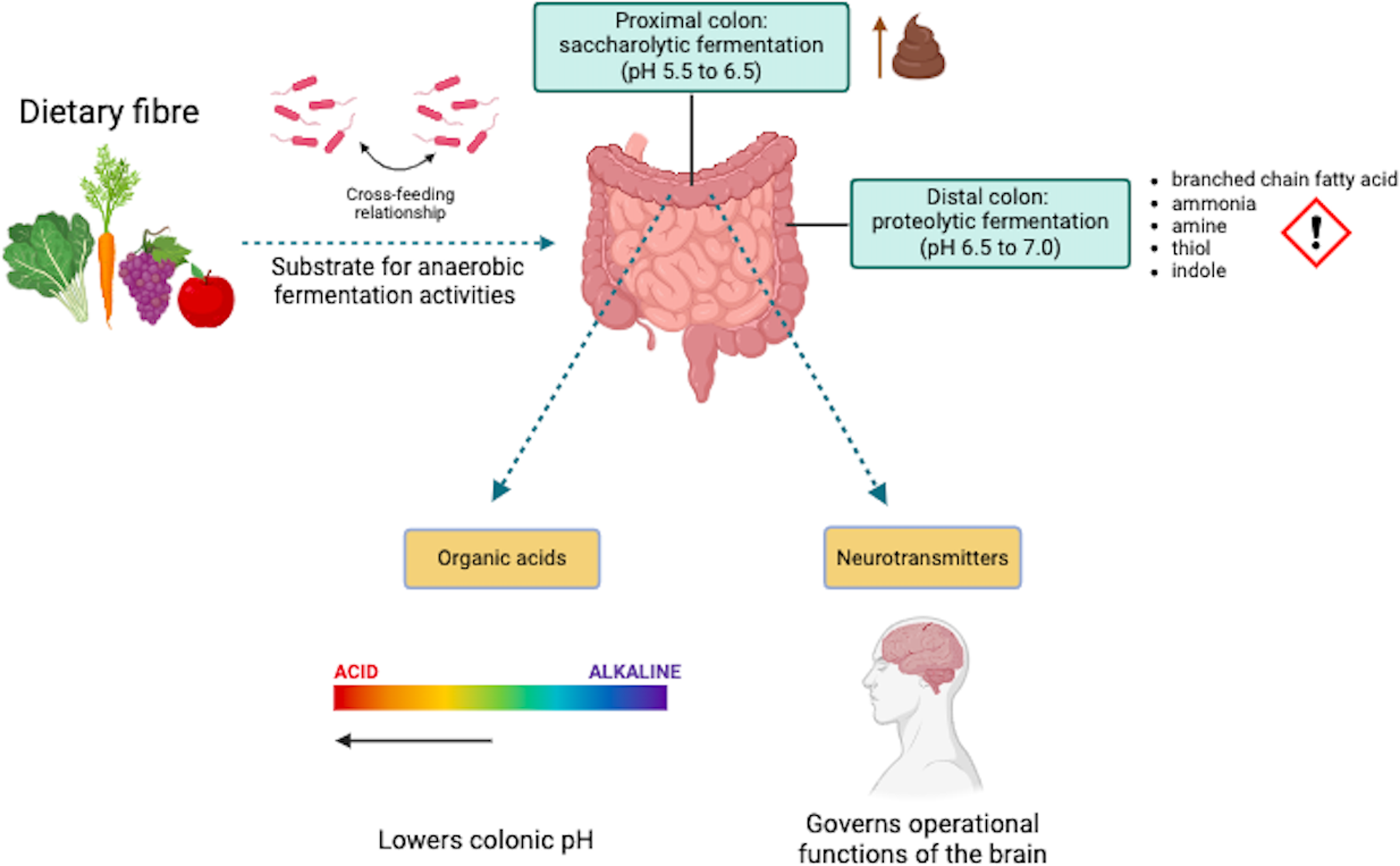
Figure 2. Metabolic activities of the gut microbiota. Created with BioRender.com.
The degradation of DF by the gut microbiota provides substrates supporting a symbiotic relationship with the human host and other microbes(Reference Williams, Grant and Gidley9,Reference Holscher45) . Gut microbial species utilise energy from the degradation process for survival, growth, reproduction, provision and maintenance of cellular functions for the human host(Reference Cryan and Dinan14,Reference Browne, Neville and Forster75) . Highly specialised microbial species that degrade DF directly are referred to as primary degraders or keystone species(Reference Deehan, Duar and Armet64,Reference Cronin, Joyce and O’Toole76) . Degradation of DF by these keystone species results in partial breakdown products, including SCFA, which can lower the colonic pH(Reference Holscher45,Reference Coyte, Schluter and Foster77) . This acidic condition can benefit the primary degraders and potentially other microbial species. However, it may also inhibit the growth of another microbial species(Reference Holscher45,Reference Coyte, Schluter and Foster77) . For example, the lower colonic pH promotes butyrate producers that thrive under acidic conditions and reduces acid-sensitive species such as members of the Bacteroides genus(Reference Holscher45). Additionally, SCFA produced during DF degradation can affect neurotransmitter levels(Reference Silva, Bernardi and Frozza16,Reference Gershon and Tack78) , such as serotonin, highlighting the link between gut microbial activity and brain function.
Further, secondary degraders taking up metabolites released by primary degraders(Reference Sung, Kim and Cabatbat79) is generally termed ‘cross-feeding’(Reference Smith, Shorten and Altermann80). This process can occur within the same species, between different species or in a complex system where species depend on each other(Reference Mee, Collins and Church81,Reference Hoek, Axelrod and Biancalani82) . A cross-feeding relationship can be observed in a co-culture experiment where Ruminococcus bromii, a primary degrader, metabolises RS type 2 and RS type 3, which subsequently stimulates metabolite utilisation by species Eubacterium rectale, Bacteroides thetaiotaomicron and Bifidobacterium adolescentis (Reference Ze, Duncan and Louis70). Therefore, DF degradation products are part of a complex web of interactions that influences the symbiotic relationship between the gut microbiota and the human host highlighting the crucial role of this ecosystem in human health.
Organic acids
The degradation of DF by microbial species produces organic acids(Reference Holscher45,Reference Coyte, Schluter and Foster77) . Among the organic acids are SCFA that are saturated fatty acids containing aliphatic carboxylic acid tails of up to six carbon atoms(Reference Akhtar, Chen and Ma83). In ascending order of carbon atoms, the six SCFA are formate, acetate, propionate, butyrate, valerate and caproate(Reference Akhtar, Chen and Ma83). Acetate, propionate and butyrate are the primary SCFA as they are produced at a higher rate than other SCFA(Reference Blaak, Canfora and Theis8,Reference O’Grady, O’Connor and Shanahan22,Reference Heras, Melgar and MacSharry84) .
SCFA are often measured from faecal or plasma samples(Reference Boets, Gomand and Deroover85). However, their levels may vary depending on the production and absorption into gut epithelial cells. Acetate concentrations then propionate and butyrate concentrations are considered more prevalent, as they are utilised by colonocytes(Reference Williams, Grant and Gidley9,Reference O’Grady, O’Connor and Shanahan22,Reference Alonso and Guarner86) , taken up by hepatocytes(Reference Williams, Grant and Gidley9,Reference O’Grady, O’Connor and Shanahan22,Reference Tan, McKenzie and Potamitis87) , enter peripheral circulation(Reference Williams, Grant and Gidley9). Therefore, it has been suggested that the concentrations of SCFA in faecal and plasma samples might not accurately represent their in vivo production(Reference Boets, Deroover and Houben88).
Recent studies found that colonic transit time affects SCFA production(Reference Müller, Hermes and Canfora89). A longer transit time in the descending colon was associated with lower plasma acetate concentrations but not butyrate or propionate concentrations(Reference Müller, Hermes and Canfora89). More SCFA are released from the distal colon into the circulation compared to the proximal colon(Reference Boets, Deroover and Houben88), potentially due to greater proximal gut mucosal metabolism(Reference Topping and Clifton90). Additional factors include variations in SCFA production by the microbiota between proximal and distal colon(Reference Thursby and Juge1) and the differences in apical and basolateral sides of epithelial cells uptake and transport across gut segments(Reference Cummings, Pomare and Branch91). Therefore, faecal SCFA levels are suggested to primarily reflect production and/or absorption in the distal colon rather than the proximal colon(Reference Boets, Gomand and Deroover85). Nonetheless, the production and absorption of SCFA are dynamic. These processes can change based on factors, including the consumption of different types and doses of DF(Reference Heras, Melgar and MacSharry84) and the presence of specific microbial species capable of metabolising the DF(Reference Power, O’Toole and Stanton13).
Neurotransmitters
The gut microbiota also produces neuroactive metabolites, including neurotransmitters such as serotonin, which influence gut motility, secretion and neurological functions related to behaviour and mood(Reference Gershon and Tack78,Reference Sun, Cheng and Zeng92–Reference Strandwitz96) . Serotonin is primarily produced by enterochromaffin cells in the gut(Reference Gershon and Tack78), with tryptophan, a dietary amino acid, as a precursor(Reference Kałużna-Czaplińska, Gątarek and Chirumbolo97). Tryptophan metabolism and serotonin production are also modulated by specific commensal microbes(Reference Kałużna-Czaplińska, Gątarek and Chirumbolo97) that degrade tryptophan or convert it into serotonin via tryptophan synthetase enzyme(Reference O’Mahony, Clarke and Borre98). These include genera such as Clostridium, Ruminococcus, Blautia, Lactobacillus (Reference Williams, Van Benschoten and Cimermancic99), Lactococcus, Streptococcus, Klebsiella and species like Escherichia coli (Reference O’Mahony, Clarke and Borre98,Reference Martin, Osadchiy and Kalani100) .
Dietary fibre may influence serotonin pathways indirectly by shaping the composition and metabolic activity of these microbes. Microbial degradation of DF produces SCFA, which help maintain gut barrier integrity and modulate immune responses(Reference Holscher45,Reference Coyte, Schluter and Foster77) , thereby influencing tryptophan metabolism and serotonin production(Reference Oleskin and Shenderov17,Reference Yano Jessica, Yu and Donaldson Gregory101) . Low DF intake and the resulting dysbiosis have been linked to disrupted serotonin signalling, especially in disorders of gut-brain interaction(Reference Chang, Chang and Starnes102–Reference Martel104) such as IBS(Reference Stolfi, Maresca and Monteleone33,Reference Gershon and Tack78) , which frequently co-occur with anxiety and depression(Reference Zamani, Alizadeh-Tabari and Zamani39). For instance, mice lacking serotonin reuptake transporter in the gut mucosal cells exhibit alternating bouts of diarrhoea and constipation(Reference Chen, Li and Pan103). In humans, reduced expression of this transporter has been reported in individuals with IBS or inflammatory bowel disease(Reference Coates, Mahoney and Linden105), potentially leading to increased mucosal serotonin exposure and desensitisation of serotonin receptors(Reference Coates, Mahoney and Linden105). Subsequently, these reduce reflex activity, luminal secretion and gut motility(Reference Coates, Mahoney and Linden105). In the brain, dysfunction of the serotonin reuptake transporter is associated with mood disorders(Reference Jenkins, Nguyen and Polglaze106), reinforcing the gut-brain interaction influenced by DF-microbiota interactions.
Moreover, SCFA can affect neurotransmitter levels(Reference Silva, Bernardi and Frozza16,Reference Gershon and Tack78) , including serotonin(Reference Oleskin and Shenderov17,Reference Yano Jessica, Yu and Donaldson Gregory101) . For instance, propionic acid and butyric acid help regulate host gut cell gene expression(Reference Clarke, Stilling and Kennedy107), which can have downstream effects on neurotransmitter synthesis. However, it remains a challenge to understand whether these changes in SCFA levels are directly associated with the development of diseases or are a consequence of disease-related changes in the gut microbiota(Reference Long, Gahan and Joyce108) or whether they are primarily diet-dependent(Reference Chen, Wang and Kuo7).
Together, these findings suggest that DF can modulate neurotransmitter signalling via gut microbiota mediated pathways. This highlights the potential of DF-based strategies to modulate the gut microbiome and psychological well-being.
Impact of dietary fibre interventions on the gut microbiome
Different types of DF induce varied changes in the gut microbiome, whether post-intervention compared to baseline and/or between intervention groups. Most studies from more than a decade ago used a metagenomic approach, while one recent study used a culture-based experiment to explore the microbial community following asafoetida-curcumin complex in turmeric capsules administration(Reference Amalraj, Varma and Jacob109). It is not surprising that this culture-dependent study only explored a small subset of bacteria strains, specifically taxa from Bifidobacterium and Lactobacillus genera, due to the limited capability of the technique to explore a broader spectrum of bacterial strains and detect fine detail changes in the gut microbiota composition(Reference Power, O’Toole and Stanton13). Nonetheless, this study showed that a asafoetida-curcumin complex intervention increased the abundance of Bifidobacterium and Lactobacillus genera compared to the control group(Reference Amalraj, Varma and Jacob109).
However, studies using metagenomics of faecal samples found varied impact of DF and prebiotics consumption. Following a mixed fibre intervention changes in gut microbiota composition occurred as rapidly as four days(Reference Tian, Zhang and Luo110). However, in other studies, the relative abundances of microbial species changed either after seven days of a low fermentable oligo-, di- and mono-saccharides and polyols (FODMAP) diet and oligofructose(Reference Sloan, Jalanka and Major111) or after four weeks of prebiotic oligofructose and prebiotic candidate 2’fucosyllactose(Reference Jackson, Wijeyesekera and Williams112). An intervention with GOS also showed increased Shannon (alpha) diversity and relative abundances of Bacteroidetes, Clostridia and Bifidobacterium genera compared to the control group(Reference Johnstone, Milesi and Burn113). Similarly, a prebiotic mixture containing different prebiotics and DF increased the relative abundance of the Actinobacteria phylum and Bifidobacterium species but showed no differences in SCFA concentrations compared to the control group(Reference Kang, Tang and Walton114).
Similarly, other types of DF intervention led to mixed results on the gut microbiota. The consumption of 14 gram (g) resistant dextrin for four weeks did not affect the gut microbiota composition and predictive function(Reference Barber, Sabater and Ávila-Gálvez115). However, this result was not seen in studies with resistant maltodextrin. A three-week intake of 15 g or 25 g of resistant maltodextrin increased the relative abundance of Fusicatenibacter saccharivoran (Reference Mai, Burns and Solch116), but only 25 g of resistant maltodextrin increased Bifidobacterium counts after four weeks(Reference Burns, Solch and Dennis-Wall117) and increased that of species Akkermansia muciniphila and Faecalibacterium prausnitzii after three weeks(Reference Mai, Burns and Solch116). Further, a three-week intervention with 4·5 g chitin-glucan changed several microbial species abundances(Reference Neyrinck, Rodriguez and Zhang118,Reference Rodriguez, Neyrinck and Zhang119) and faecal SCFA concentrations, particularly butyric and caproic acids(Reference Rodriguez, Neyrinck and Zhang119). A 24-week rice bran intake increased the relative abundances of Firmicutes phylum and Lactobacillus genus compared to rice powder intake(Reference So, Chan and Law120). Similarly, there were changes in gut microbiota composition after the consumption of broccoli and daikon radish for 18 d(Reference Kaczmarek, Liu and Charron121), of crackers containing RS for 10 d(Reference Sri Lakshmi Sravani, Dorothy and Jennifer122), bread and biscuits containing refined or wholemeal amylose wheat for four weeks(Reference Gondalia, Wymond and Benassi-Evans123), a snack bar containing 7 g of chicory inulin-type fructans daily for four weeks(Reference Gondalia, Wymond and Benassi-Evans123) and biscuits enriched with olive pomace for eight weeks compared to control group(Reference Conterno, Martinelli and Tamburini124). These findings overall highlight the diverse and duration-dependent effects of different types of DF interventions on the gut microbiota.
In terms of gut microbiota diversity, there was no difference in the reviewed studies. This includes a four-week study providing either chitin-glucan(Reference Rodriguez, Neyrinck and Zhang119), rice bran(Reference So, Chan and Law120), mixed prebiotics(Reference Kang, Tang and Walton114), fruit pomace(Reference Alexander, Brauchla and Sanoshy125) or polydextrose(Reference Berding, Long-Smith and Carbia126), a two-week study using potato RS, maize RS or chicory root inulin(Reference Baxter, Schmidt and Venkataraman127), two-week of a whole grain diet(Reference Vanegas, Meydani and Barnett128), an eight-week intervention of olive pomace enriched biscuits(Reference Conterno, Martinelli and Tamburini124), a 10-week high DF diet(Reference Wastyk, Fragiadakis and Perelman129) or 18 d of cooked broccoli and daikon radish(Reference Kaczmarek, Liu and Charron121). Interestingly, a diet containing six serves or more of fermented foods increased alpha diversity, but not a diet containing 20 g or more fibre(Reference Wastyk, Fragiadakis and Perelman129). The alpha diversity was also lower following a 12-week wheat bran-derived arabinoxylan oligosaccharides intervention, possibly resulting from softer stools, selective stimulation and growth of the Bifidobacterium genus(Reference Müller, Hermes and Emanuel130). Therefore, this alpha diversity reduction might not correlate with gut microbiota instability(Reference Müller, Hermes and Emanuel130).
Overall, the reviewed studies that explored the effect of DF on the gut microbiome showed mixed results. Different study durations and washout periods, high inter-individual variability and limited research examining different DF types examined might have been contributing factors. Comparing these studies directly is challenging, which warrants better designed clinical trials. Future studies on DF should consider baseline dietary intake and individual gut microbiota composition prior to intervention. The gut microbiota of individuals with low DF intake and limited baseline microbial diversity may respond more to the intervention as compared to their counterparts(Reference Jefferson and Adolphus131). Therefore, a personalised nutrition approach may be more effective, allowing improved adherence to the dietary intervention.
Impact of dietary fibre interventions on psychological well-being
A handful of studies have explored the effects of DF on psychological well-being in healthy adults. These studies used either resistant dextrin(Reference Barber, Sabater and Ávila-Gálvez115), chitin-glucan(Reference Rodriguez, Neyrinck and Zhang119), wheat bran-derived arabinoxylan oligosaccharides(Reference Müller, Hermes and Canfora89), GOS(Reference Johnstone, Milesi and Burn113), polydextrose(Reference Berding, Long-Smith and Carbia126), snack bar containing chicory inulin-type fructans(Reference Reimer, Soto-Vaca and Nicolucci132) or a low FODMAP diet supplemented with prebiotics(Reference Mego, Manichanh and Accarino133). These studies found that the interventions did not affect well-being, quality of life or mood. However, GOS supplementation tended to decrease anxiety symptoms in participants with anxiety(Reference Johnstone, Milesi and Burn113). These findings may be influenced by a ceiling effect, where individuals with already high well-being levels have limited potential for further improvement. This suggests that the effectiveness of these interventions may depend on participant selection criteria, with greater effects potentially observable in those with lower baseline well-being(Reference Goedendorp and Steverink134,Reference Judd and Kenny135) .
Further, co-administration of a prebiotic consisting of oligofructose and 2’fucosyllactose, demonstrated a decrease in depression, anxiety and negative affect schedule scores in a double-blind, placebo-controlled, randomised controlled trial(Reference Jackson, Wijeyesekera and Williams112). Despite research on co-administering DF or prebiotics is limited, combining isolated fibres might offer a ‘dual treatment’ for gut-related and psychological outcomes(Reference Gill, Rossi and Bajka20). This was shown where the co-administration of isolated oligofructose and 2’fuscosyllactose increased the relative abundances of several beneficial butyrate-producing microbes, including taxa from the Lactobacillus and Blautia genera, after four weeks of intervention(Reference Jackson, Wijeyesekera and Williams112). The authors observed several positive correlations between several mental health scores improvement and presence of the genera Bifidobacterium, Roseburia, Anaerostipes, Blautia and the species Faecalibacterium prausnitzii in in their cohort(Reference Jackson, Wijeyesekera and Williams112). The authors speculated that gut microbiota manipulation may influence mental health by regulating neurological pathways(Reference Silva, Bernardi and Frozza16,Reference Jackson, Wijeyesekera and Williams112) . This can be explained by the ability of some DF types to promote microbes involved in serotonin precursors production or tryptophan metabolism, linking gut microbial activity to serotonin signalling and mood regulation(Reference Oleskin and Shenderov17,Reference Holscher45,Reference Coyte, Schluter and Foster77,Reference Kałużna-Czaplińska, Gątarek and Chirumbolo97–Reference Yano Jessica, Yu and Donaldson Gregory101) . Nonetheless, as these findings are based on associations rather than mechanistic evidence, further research is needed to establish causal pathways.
There is no conclusive evidence on how specific DF types or food items modulate the gut microbiota to improve psychological well-being. However, some prebiotic fibres, such as GOS, oligofructose and 2’fucosyllactose have shown promise in modulating microbial composition and mood-related outcomes, particularly among individuals with lower baseline well-being. Therefore, consuming a variety of DF-rich foods, including those high in prebiotic fibres may help support a beneficial gut microbiome and potentially improve psychological well-being.
Future directions
Determining the most suitable food or dietary pattern is crucial to help manage specific diseases or disorders. Furthermore, this may also help in designing personalised nutrition strategies to alter the gut microbiota and optimise the health and metabolism of humans(Reference Berding, Vlckova and Marx136). However, several reviews and intervention studies have suggested that factors, including season, age(Reference Davenport, Mizrahi-Man and Michelini137), both baseline gut microbiota and well-being as well as habitual dietary patterns can influence gut microbiota composition and overall response to a dietary intervention(Reference Lampe, Navarro and Hullar138,Reference Griffin, Ahern and Cheng139) .
Baseline microbial richness, diversity and stability might be able to predict dietary interventions’ effectiveness(Reference Tap, Furet and Bensaada140–Reference Hughes, Kable and Marco142). A systematic review proposed that individuals with low DF intake and limited baseline microbial diversity may show more gut microbiome changes following intervention(Reference Jefferson and Adolphus131), particularly after acute dietary interventions(Reference Cotillard, Kennedy and Kong143). However, taxonomy changes may be relatively temporary, subtle and inconsistent compared to the effects of habitual, sustained diet(Reference Wu, Chen and Hoffmann11). The authors of a review further argued that a stable gut microbiota may suggest a stable response or that there may not be a change to the diet(Reference Hughes, Kable and Marco142). An unstable gut microbiota may indicate a flexible response to the ‘optimal diet’. This may require constant re-evaluation of the diet(Reference Hughes, Kable and Marco142), which can be challenging to determine the effectiveness of the dietary intervention.
The taxonomic composition of the adult gut microbiota is relatively stable over extended periods(Reference Wu, Chen and Hoffmann11). The abundance of individual taxa may be susceptible to alterations in dietary patterns and geographic area(Reference David, Maurice and Carmody10,Reference David, Materna and Friedman144) . With the diverse dietary and lifestyle choices available, there is a tendency for each individual to have a highly individualised gut microbiome(Reference Albenberg and Wu145). Large phylum-level adjustments in response to dietary changes may be more pronounced in animal models involving rodents or pigs due to the highly controlled study environment(Reference Wu, Chen and Hoffmann11). To further substantiate this, a one-year longitudinal study of two individuals showed that 75 % to 88 % of bacteria were relatively stable for several months. However, relative abundances of specific taxa, including E. rectale, F. prausnitzii, Eggerthella, Clostridium, Ruminococcus, Blautia and Bifidobacteriales shifted within a day following a change in geographical and DF intake in one of the participants(Reference David, Materna and Friedman144). Following travel for 51 d, the gut microbiota of this participant returned to its pre-travel state within 14 d. The authors suggested that this reversal was partly due to temporarily adopting the local diet abroad(Reference David, Materna and Friedman144). Nonetheless, the range of behavioural choices was limited to these two individuals and the shift in gut microbiota was observed in only one participant(Reference David, Materna and Friedman144). In this context, migration and intervention studies will be particularly valuable in understanding the long-term impact of dietary and environmental changes on gut microbial composition, warranting further clinical research in this area.
The addition of certain types of DF may lead to unwanted negative gut symptoms. Tolerances between individuals vary, but a sudden increase or a drastic change in DF intake may result in bloating, discomfort and increased flatulence(Reference Eswaran, Muir and Chey146). These potential adverse effects should also be considered in future studies to ensure both efficacy and tolerability of DF interventions.
Conclusion
Taken together, evidence remains inconclusive on how habitual intake of DF supplementation through whole foods influence the gut microbiome and psychological well-being in adults. Key knowledge gaps include the effects of specific DF types, inter-individual variability and the impact of habitual v. acute intake. Baseline microbial composition, dietary patterns and psychological status may influence dietary intervention outcomes, highlighting the potential for stratified or personalised approaches. To address this, there is a pressing need for larger and well-powered clinical trials that incorporate longitudinal biological sample collections, advanced sequencing techniques and other -omics techniques (including novel dietary biomarkers and microbial metabolites), validated subjective questionnaires and comprehensive dietary records. Integrating these data can enhance understanding of the gut microbiome, while mechanistic studies driven by clinical observations are essential for identifying underlying biological pathways. These insights may ultimately support the development of targeted interventions that can be applied to the general public and clinical practices.
Acknowledgments
The authors would like to thank the Nutrition Society for inviting Catherine Wall to present at the 2024 Nutrition Society of New Zealand held at the University of Otago, Christchurch Campus, New Zealand and for the review invitation.
Financial support
H.M.N. was a PhD student at the time of the original work, which was part of her PhD thesis and was supported by the University of Otago Doctoral Scholarship and the High-Value Nutrition National Science Challenge. No external financial support was received for the revision phase of this manuscript.
Competing interests
There are no conflicts of interest.
Authorship
H.M.N. wrote the paper and designed the figures. C.L.W., S.B.B., R.B.G. and N.C.R. critically read the paper and assisted in the revision of the content. All authors approved the final version of the manuscript.





