This abstract was presented as the Whole Body Metabolism Theme highlight.
Lifestyle intervention studies in overweight and obese pregnancy have reported limited success regarding improvements in important pregnancy and neonatal outcomes(Reference Oteng-Ntim, Varma and Croker1). Improved understanding of how maternal weight status and nutrition in pregnancy influence underlying metabolic pathways may reveal optimal targets for more effective future interventions. Fatty acid profiles may be particularly relevant in this regard due to associations with maternal glucose intolerance(Reference Chen, Scholl and Leskiw2) and excess offspring adiposity(Reference Moon, Harvey and Robinson3). This study aims to investigate the associations of maternal pre-pregnancy body mass index (pBMI), GWG and dietary intake on maternal plasma non-esterified fatty acids (NEFA) across pregnancy.
Non-diabetic pregnant women (N = 160) were recruited in early pregnancy and followed prospectively with research laboratory visits in each trimester. Each visit included fasting blood samples, maternal self-reported pre-pregnancy weight, measured weight and height. Dietary intake was assessed by the 24-hr recall method over three non-consecutive days in each trimester, including one weekend day. Average daily trimester values were computed for total energy intake and the Alternative Healthy Eating Index adapted for pregnancy (AHEI-P), as a validated measure of dietary quality(Reference Rodriguez-Bernal, Rebagliato and Iniguez4). GWG from pre-pregnancy up until each trimester was calculated and pBMI (kg/m2) was determined using average values for pre-pregnancy weight and measured height. A targeted LC-MS/MS metabolomics platform determined 21 NEFA metabolites as previously described(Reference Lindsay, Hellmuth and Uhl5). The association of each NEFA with pBMI, GWG, energy intake and AHEI-P were analysed by multiple linear regression, adjusting for maternal age and ethnicity. Bonferroni correction accounted for multiple testing.
Mean ± SD pBMI was 25·9 ± 6·0 Kg/m2 and GWG from pre-pregnancy to trimester 3 was 10·2 ± 5·3 Kg. Mean AHEI-P score increased slightly between trimester 1 (54·9) and 3 (56·6), but with large variation observed (SD = 11). 42 % of women were classified overweight or obese. pBMI was independently associated with elevated NEFA, particularly in trimester 2 (Fig 1). Omega-3 NEFA C20:5 and C22:6 were unaffected in any trimester, while the omega-6 NEFA C20:3, 20:4 and 22:4 remained positively associated with pBMI throughout gestation. GWG, energy intake and AHEI-P were not associated with NEFA in any trimester.
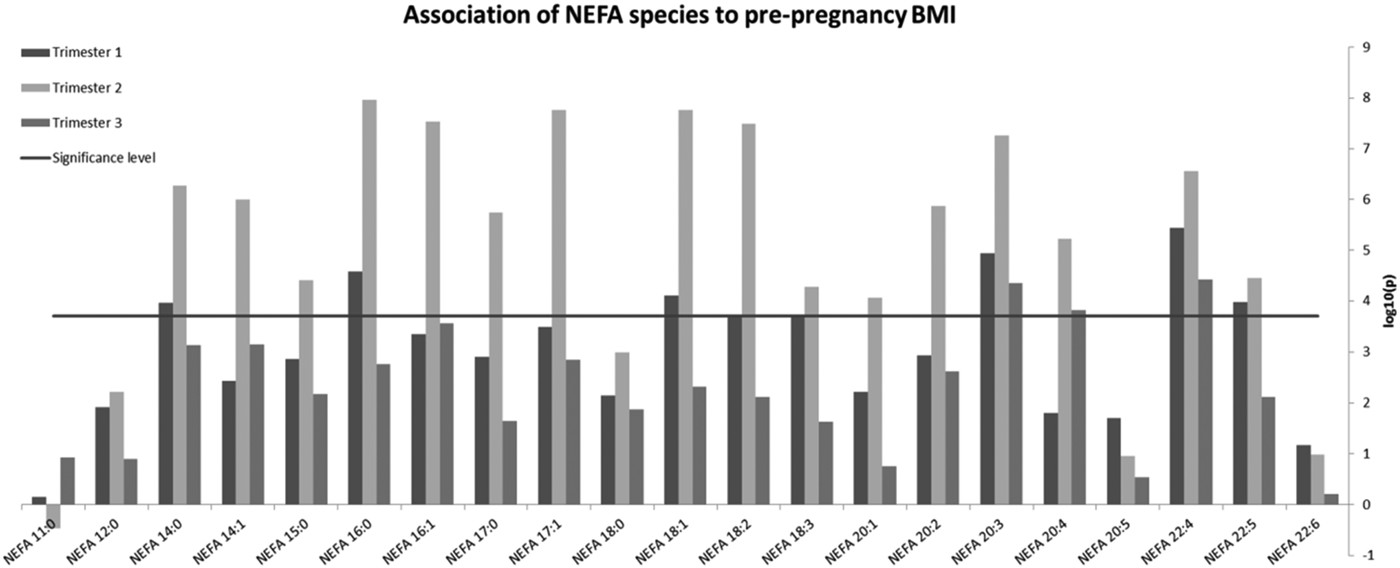
Fig. 1. Association of NEFA species to pre-pregnancy BMI.
Raised pBMI appears to particularly drive elevated levels of omega-6 NEFA, which have been implicated in fetal programming of offspring adiposity3. Pre-conception obesity may exert an over-riding influence on maternal fatty acid metabolism, regardless of variations in GWG or quality of the diet. This highlights the importance of pre-conception interventions.



