Introduction
Gastrointestinal parasites significantly cause infection and reduce welfare in small ruminants (Oliveira et al. Reference Oliveira, Crotti, Crotti, Silva, Lopez and Campana2017). In tropical and subtropical regions, Haemonchus contortus is the most prevalent nematode affecting ruminants, leading to apathy, lesions in the intestinal mucosa, and anemia (Zajac and Garza Reference Zajac and Garza2020). The second most frequent gastrointestinal nematode of sheep in Brazil is Trichostrongylus colubriformis, which causes weight loss, morbidity, and reduced food intake (Carvalho et al. Reference Carvalho, Neves, Pennacchi, Castilhos and Amarante2021). Although several diagnostic methods are available, parasite control predominantly relies on the preventive use of anthelmintics (Molento et al. Reference Molento, Pontarolo, Pires, D’allanesse, Vieira, Brandao and Aquino2021). This strategy has led to the selection of multidrug resistance to H. contortus (Amaral et al. Reference Amaral, Guerra, Santana, Chicoy, Arenal, Lima, Alves, Molento and Faustino2021), prompting research into new active compounds (Bortoluzzi et al. Reference Bortoluzzi, Buzatti, Chaaban, Pritsch, Dos Anjos, Cipriano, Deschamps and Molento2021; Selzer and Epe Reference Selzer and Epe2021; Soldera-Silva et al. Reference Soldera-Silva, Seyfried, Campestrini, Zawadzki-Baggio, Minho, Molento and Maurer2018).
Several studies on humans and animals have tested active plant components to control parasites (Chaaban et al. Reference Chaaban, Richardi, Carrer, Brum, Cipriano, Martins, Silva, Deschamps and Molento2019; Garbin et al. Reference Garbin, Munguia, Saldana, Deschamps, Cipriano and Molento2021). Esters are plant derivatives constituting the volatile base of essential oils (EO), terpenes, and other compounds. These compounds often have an intensely sweet and fruity odor, particularly during peak production when plants fully bloom (Elsharif et al. Reference Elsharif, Banerjee and Buettner2015). Linalyl acetate (LA) is an ester derived from linalool, extracted from Lavandula angustifolia EO through supercritical carbon dioxide extraction and from L. officinalis via steam distillation (Lis-Balchin et al. Reference Lis-Balchin, Deans and Eaglesham1998; Altun and Yapici Reference Altun and Yapici2022). A substantial amount of LA is obtained from L. angustifolia (79.8%) and L. officinalis (34.9%) EO. Moreover, LA exhibits analgesic, anti-inflammatory, anxiolytic, antimicrobial, and sedative properties and is effective against burns and common skin lesions (Cavanagh and Wilkinson Reference Cavanagh and Wilkinson2002). However, LA has yet to be tested against the nematodes H. contortus and T. colubriformis.
Hormesis is an adaptive stress response (Jentho et al. Reference Jentho, Lajqi, Yang, Winkler, Stojiljkovic, Wetzker and Bauer2018) that is highly relevant to explaining biological organisation and risk assessment in toxicology, microbiology, medicine, and public health (Calabrese Reference Calabrese2018). It refers to a biphasic pharmacological response where initial drug doses elicit a maximal effect. In contrast, higher doses result in a diminished/suppressive outcome, potentially reaching low or zero efficacy (Calabrese Reference Calabrese2018). A hormesis curve for plant compounds has been documented; for instance, Papanastasiou et al. (Reference Papanastasiou, Bali, Ioannou, Papachristos, Zarpas and Papadopoulos2017) reported that limonene induced a typical hormetic insecticidal effect against the Mediterranean fruit fly Ceratitis capitata. This study aimed to determine the motility effect of LA against larvae of Haemonchus spp. and Trichostrongylus spp. and to report the hormetic effect of LA on these nematodes.
Methodology
Plant material and nematode parasites
Pure LA was acquired from Quinari (Ponta Grossa, Brazil), reference 1331-1, produced by vapor pressure of 0.111 mm Hg at 25°C. The component was extracted from the flowers of L. officinalis Mont Blanc. The material was kept in a capped amber bottle until testing, when it was solubilised in distilled water containing 2% v/v Tween 80.
Fecal samples were collected from four naturally infected male White Dorper sheep from a farm located in Pinhais, Brazil (-25.38638, -49.12646). The material was subjected to a modified coprological technique (Gordon and Whitlock Reference McL and Whitlock1939) for quantifying nematode eggs, using 4 g of feces in 26 ml of saturated sucrose solution, and the result was multiplied by 25. Subsequently, fecal samples were mixed with an equal volume of vermiculite, moistened, and incubated at 25°C for 10 days to obtain third-stage larvae (L3). After that, the coproculture was then placed in a Baermann apparatus to collect L3. All larvae were identified at the genus level, according to van Wyk and Mayhew (Reference van Wyk and Mayhew2013).
The project was approved by the Animal Ethics Committee (CEUA) of the Agricultural Sciences Sector of the Federal University of Paraná, protocol 001/2019.
Larval migration inhibition test – LMT
The LMT was employed to determine the effect of LA on L3 following the methodology described by Demeler et al. (Reference Demeler, Küttler and von Samson-Himmelstjerna2010) with modifications. L3 were incubated in 2-ml opaque airtight microtubes. Approximately 200 L3 were incubated in the presence of LA at concentrations of 0.89, 2.24, 4.47, 8.95, 17.9, 35.8, 71.6, and 143.2 mg/ml. After the first incubation of 18 h, the total content of each tube was transferred to a 24-well plate where a 25 μm mesh separated two environments (upper and lower). The plate was sealed with parafilm to prevent volatilisation and incubated for another 24 h at 28°C under constant light. Subsequently, the mesh was carefully removed, and the L3 were counted under an inverted Optiphase INV-403 microscope (van Nuys, USA).
Statistical analysis
The percentage of L3 that did not migrate was determined for all controls and from each LA concentration. Each sample was tested in four replicates, including a negative control of distilled water (NC), a control using 2% Tween 80 (CT), and a positive control using 20% nitroxynil (C-NTX). A nonlinear regression analysis of the response to the concentrations was performed. Pharmacological comparison and efficacy were determined on a log scale (X = log X). A four-parameter logistic equation with variable slope was chosen using GraphPad Prism 6.1 (San Diego, USA). The efficacy was calculated according to the following formula:
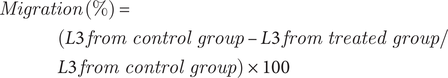 $$ {\displaystyle \begin{array}{l}\hskip-5em Migration\;\left(\%\right)=\\ {}(L3\; from\ control\ group\hbox{--} L3\; from\ treated\ group/\\ {}L3\; from\ control\ group)\times 100\end{array}} $$
$$ {\displaystyle \begin{array}{l}\hskip-5em Migration\;\left(\%\right)=\\ {}(L3\; from\ control\ group\hbox{--} L3\; from\ treated\ group/\\ {}L3\; from\ control\ group)\times 100\end{array}} $$
Results and discussion
L3 identification before testing revealed the presence of Haemonchus spp. (47.7%) and Trichostrongylus spp. (38.5%). As parasite populations were collected from the same farm with no previous drug resistance record, we assume a similar distribution after incubation. The LMT indicated that the three lowest concentrations of LA exhibited a concentration-dependent effect (stimulation) on the larvae with a linear correlation of 0.950 (Table 1). At doses of 8.95 and 17.9 mg/ml, the inhibition of L3 motility was 49% and 59% (plateau), respectively. At concentrations of 35.8, 71.6, and 143.2 mg/ml, the results showed a steady and proportional decrease in L3 migration inhibition of 44.7, 28.3, and 5.2%, respectively. The NC and CT did not affect L3 migration. NTX exhibited 91.5% efficacy (Table 1). According to Bosly (Reference Bosly2022), in Saudi Arabia, the knockdown rate of lavender oil at 5%, containing 6.7% LA, was 95.5% in adults of Culex pipiens. In larvae, lavender oil induced an 87.2% mortality after 60 minutes of exposure (LC50 = 301.1 ppm). Mantovani et al. (2013) demonstrated 100% activity of 200 μg/ml of LA against adult forms of Schistosoma mansoni in Australia. The LC50 values against S. mansoni at 24 and 120 h incubation were 117.7 and 103.9 μg/ml, respectively.
Table 1. Percentage (%) of nematode larval inhibition of migration and standard deviation (SD) after treatment with linalyl acetate (LA)
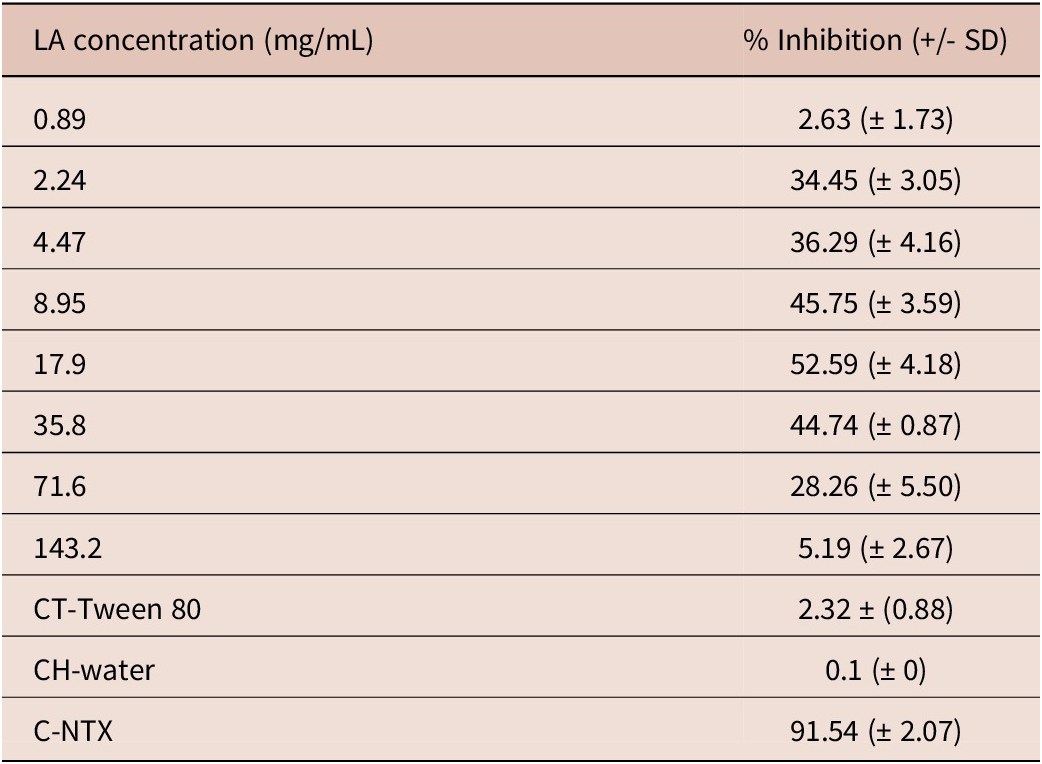
Figure 1 illustrates the hormetic effect of LA, demonstrating an inverted U-shaped curve characterised by an increase, plateau, and subsequent decrease in efficacy. The data suggest that at higher concentrations, LA promotes an opposing mechanism of action, blocking the parasite’s receptors to the compound and preventing future interactions (Calabrese and Mattson Reference Calabrese and Mattson2017). The hormetic mechanism may be based on the premise that low stress activates or positively induces responses to the molecules. The hormetic effect may also depend on the organism’s nutritional status, temperature, and other preconditions (Calabrese Reference Calabrese2018).
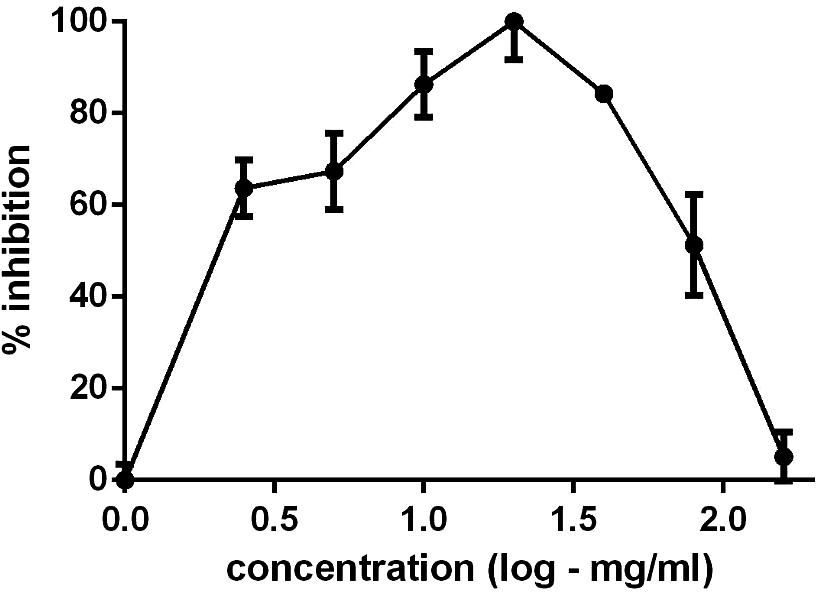
Figure 1. Hormetic concentration-dependent effect of linalyl acetate against Haemonchus spp. and Trichostrongylus spp.
It is well known that the biological effect of EO depends on the presence or absence of various components (Mande and Sekar 2020). The hormetic effect of EO has been described in Drosophila melanogaster (fruit fly), Musca domestica (housefly), and Acheta domesticus (cricket) (Cutler et al. Reference Cutler, Amichot, Benelli, Guedes, Qu, Rix, Ullah and Desneux2022). The EO of Rosmarinus officinalis (rosemary), which contains 27.5% of α-pinene in fully ripe fruits, stimulated the growth of radish roots and aerial parts at doses up to 300 μl/L but decreased the stimulus at doses greater than 1200 μl/L (Alipour and Saharkhiz Reference Alipour and Saharkhiz2016). When used against the adult stage of Ceratitis capitata (Benakeio fly), limonene increased the fertility and life expectancy of the species in low concentrations, but these effects diminished considerably at higher doses (Papanastasiou et al. Reference Papanastasiou, Bali, Ioannou, Papachristos, Zarpas and Papadopoulos2017). Citrus myrtifolia (Chinotto orange) exhibited a hormetic impact on Lolium multifolium (Italian ryegrass) seeds, containing 76.8% limonene and 1.0% LA (Caputo et al. Reference Caputo, Cornara, Bazzicalupo, de Francesco, de Feo, Trombetta and Smeriglio2020). The hormetic effect was also observed in A375 cells during tests with an aqueous emulsion of bergamot (Citrus bergamia) and orange (C. sinensis) EO containing 43.3% limonene (Alexa et al. Reference Alexa, Galuscan, Soica, Cozma, Coricovac, Borcan, Popescu, Mioc, Szuhanek, Adriana Dehelean and Jumanca2022). Thymus vulgaris (thyme), which contains thymol as the main component (33%), also showed a hormetic effect on UMSCC1 human cancer cells with a decrease in its lethal effect at concentrations above 0.54 mg/ml (Sertel et al. Reference Sertel, Eichhorn, Plinkert and Efferth2011).
A crucial issue also relates to the ecological relevance of hormesis in an incorrect drug resistance diagnostic. Drug resistance is an evolutionary phenotypic response represented by a lack of parasite reduction (i.e., H. contortus) (Silva et al. Reference Silva, Amaral, Ferraudo and Silva2022) manifested by parasite paralysis and death. Under a hormetic effect, this lack of reduction may not demonstrate resistance, as organisms might moderately adapt their behavior or even increase their fitness in response to changing environments (Costantini Reference Costantini2019; Jalal et al. Reference Jalal, de, Ribeiro, Fernandes, Mariano, Trindade and Reis2021). Kishimoto et al. (Reference Kishimoto, Uno, Okabe, Nono and Nishida2017) reported the transmission of a resilient phenotype for oxidative stress and proteotoxicity through hormetic mechanisms in Caenorhabditis elegans. The hormetic effect was transmitted epigenetically upon exposure to various stressors during developmental stages, providing survival advantages for generations after controlled stress exposure. Therefore, the inverted U-shaped efficacy of a phytochemical component against two species of ruminant parasites observed in vitro should be taken with caution, as reports of lack of effectiveness are a consequence of two major stress factors of frequent drug use, and the combination of products (Molento and Brandão Reference Molento and Brandão2020). This distinctive stimulation-and-inhibition effect should also be considered when selecting new compounds for preclinical testing.
Conclusion
LA was effective against free-living stages of Haemonchus spp. and Trichostrongylus spp. However, the compound exhibited hormetic activity at doses above 35.8 mg/ml, demonstrating a reduction in efficacy to only 5% at 143.2 mg/ml. This study is the first to report the hormetic effect of a phytochemical component against nematode parasites of ruminants.
Acknowledgements
The authors are thankful for the financial support from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, CAPES, grant 2016/02, and Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq, grant 001/2018, Brazil.
Competing interest
The authors declare no conflict of interest.




