Introduction
Patent ductus arteriosus is the most common cardiovascular derangement of premature infants, with an incidence inversely correlating with the gestational age, affecting up to 80% of infants born weighing less than 1200 g. Reference Dice and Bhatia1 Haemodynamically significant patent ductus arteriosus is associated with decreased survival and increased risks of various comorbidities. Reference Noori, McCoy and Friedlich2–Reference Gentle, Travers, Clark, Carlo and Ambalavanan4 While medical treatment is typically attempted first, it is not universally effective and is not risk-free. Reference Ashrafi and Levy5,Reference Van Overmeire, Smets and Lecoutere6 Although surgical ligation boasts a high success rate, it is associated with numerous short and long-term complications. Reference Ashrafi and Levy5,Reference Tashiro, Wang, Sola, Hogan, Neville and Perez7–Reference Clement, El-Hakim, Phillipos and Coté10 Transcatheter patent ductus arteriosus closure is quickly spreading due to its proven effectiveness and favourable complication profile. Reference Sathanandam, Justino, Waller, Radtke and Qureshi11–Reference Rahde Bischoff, Backes, Weisz, Mohammad Nijres, McNamara and Kluckow19
Historically, the limited commercial availability of suitable devices restricted the attractiveness of transcatheter closure in premature infants. Initially, devices for older children, such as coils and plugs, were explored. However, these devices faced challenges due to the need for arterial access and/or a stiff and large delivery system. Reference Zahn, Peck and Phillips14 A few centres started performing transcatheter closures in premature infants with the advent of the microvascular plug (Medtronic, Minneapolis, MN), which has a small profile allowing delivery through a soft and small delivery system. Reference Sathanandam, Justino, Waller, Radtke and Qureshi11,Reference Wang-Giuffre and Breinholt12 The revolution, though, was ignited when the Food and Drug Administration approved the Piccolo (Abbot, Santa Clara, CA) device in 2019. This device is usually placed through single venous access without requiring arterial access. Reference Sathanandam, Gutfinger and O’Brien17–Reference Dalby, Shibbani and Mercadante21
During the same year, the “KA micro plug” (KA Medical, Minneapolis, MN) was approved for vascular closure but not specifically for patent ductus arteriosus in premature infants. This device has many promising features suitable for patent ductus arteriosus closure in premature infants; such as being short, soft, and having a small profile. Despite its promising features, limited information exists about its use in infants weighing less than 1500 g. Reference Heyden, El-Said, Moore, Guyon, Katheria and Ratnayaka15,Reference Guyon, Duster and Katheria16,Reference Barry, Gudausky and Balzer20
This study describes our institution’s experience with the KA micro plug device in closing the patent ductus arteriosus of premature infants weighing less than 1500 g. It also provides insights into the steps for safe closure and a comprehensive comparison with the Piccolo device.
Material and methods
Study design
This is a retrospective, single-centre study of infants born prematurely who underwent attempted transcatheter patent ductus arteriosus closure using a KA micro plug device at Stead Family Children’s Hospital/University of Iowa from February 2022 through December 2023. Patients were excluded if their weight at the time of the procedure was equal to or exceeded 1500 g. The primary aim was to detail the procedural technique and report immediate and midterm outcomes.
Collected data
Demographic data included gestational age, birth weight, weight, and corrected gestational age at intervention. Procedural data included procedure time, radiation time, radiation dose, ductus arteriosus dimensions, device size, number of used devices, and procedural outcomes. The procedure was defined as successful if the patient left the cardiac catheterisation lab alive with a device in place. Iatrogenic left pulmonary artery stenosis and aortic obstruction are defined as the peak instantaneous pressure gradient measured by echocardiogram greater than 20 mmHg. Major procedural haemodynamic instability was defined by the need for chest compression, cardioversion, removal of equipment outside the heart for hypotension or desaturation, or aborting the procedure. Minor haemodynamic instability was defined by the need for inotropic support during the procedure. Acute and midterm follow-up data were collected including mortality, cardiac function, residual shunt, a gradient across the left pulmonary artery and the aortic arch, and the severity of tricuspid valve regurgitation.
Statistical analysis
Descriptive statistical analysis was conducted using the SPSS Statistics package [ver. 28.0.11(14)]. Categorical data are presented as numbers and percentages, while continuous data are expressed as median and range.
Device description
The KA micro plug device is constructed from a thin nitinol wire to make three equal-sized thin discs. The distal and proximal ends of the device have radiopaque bands to enhance fluoroscopy visualisation. The device is available in 4 different disc sizes: 3, 4, 5, and 6 mm. It comes in only one short unconstrained length (2.5 mm). The delivery wire of the device is thin and the distal end of the wire is softer than the rest of the wire. The device’s special design allows it to be loaded inside a 2.9-Fr microcatheter, which is provided with the package. The microcatheter is 125 cm in length and hydrophilically coated. The distal tip of the catheter has a radiopaque marker. Also, the manufacturer provides two short “Y-connectors.”
Procedure description and delivery system modification
At the start of the procedure, a brief echocardiogram is performed to confirm the presence and the size of the patent ductus arteriosus, flow pattern in the left pulmonary artery and aortic arch, cardiac systolic function, and determine the presence and degree of tricuspid valve regurgitation.
Before obtaining access, the 4-Fr 65 cm Angled Glide catheter (Terumo, Somerset, NJ) total length is denoted with a marker on the back table drape, as shown in Figure 1A.
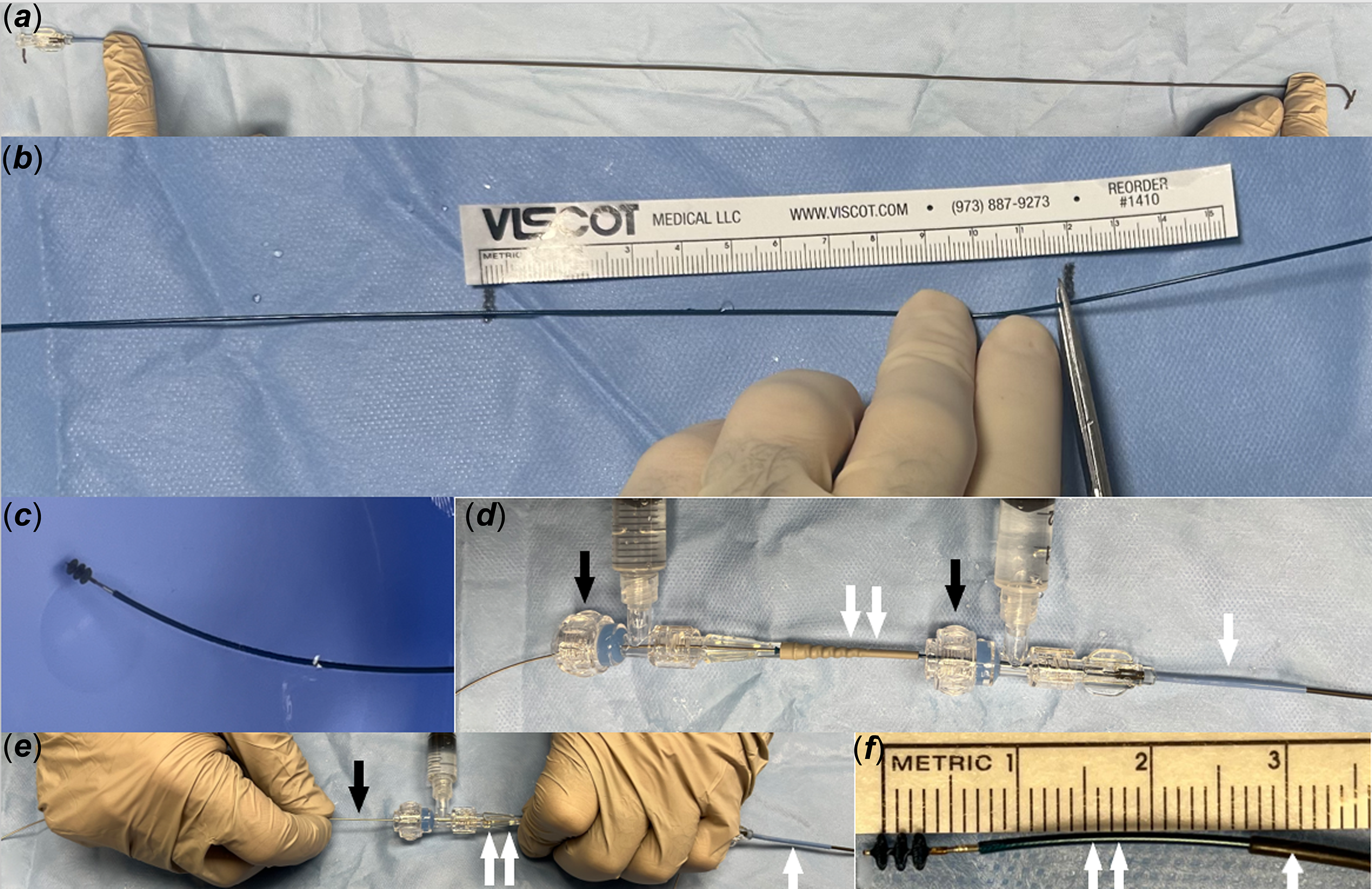
Figure 1. Images demonstrate the steps of using the KA micro plug. A. Marking the Angled Glide catheter length on the drape of the back table. B. Cutting the microcatheter 12 cm longer than the Angled Glide catheter. C . Front loading and de-airing the KA device inside a heparinised saline bowel. D. Pulling the Angled Glide catheter (single white arrow) over the microcatheter (double white arrows) to expose the distal end of the microcatheter. Notice that the Y-connectors (black arrows) are connected with the Angled Glide catheter and the microcatheter. E. Fixing the Angled Glide catheter (single white arrow) and the microcatheter (double white arrows) with the left hand and fixing the delivery wire (black arrow) with the left hand. The device is deployed by pulling both catheters as one unit over the device wire. F. Showing the KA micro plug device is deployed. Notice that when the Angled Glide catheter (single white arrow) is pulled over the microcatheter (double white arrow) as shown in “D,”, the microcatheter extends approximately 2 cm outside the distal end of the Angled Glide catheter.
Venous access is obtained in the common femoral vein using a 4-Fr sheath. The combination of a 4-Fr Angled Glide catheter and a 0.035” Tiger wire (Abbott Cardiovascular, Plymouth, MN) is inserted in the femoral venous sheath. Under fluoroscopic guidance, the patent ductus arteriosus is crossed prograde from the main pulmonary artery into the descending aorta. The Tiger wire is removed and the catheter is withdrawn inside the ductus arteriosus. An angiogram is obtained using 1 ml of non-diluted contrast. The Angled Glide catheter is advanced into the descending aorta with the help of the Tiger wire. Angiographic patent ductus arteriosus dimensions (aortic end, narrowest diameter, and length) are measured to aid in choosing the KA device size and deciding on the deployment method, as shown in Figure 2A. The device chosen is 1.2–2 mm larger than the narrowest ductus arteriosus dimension.
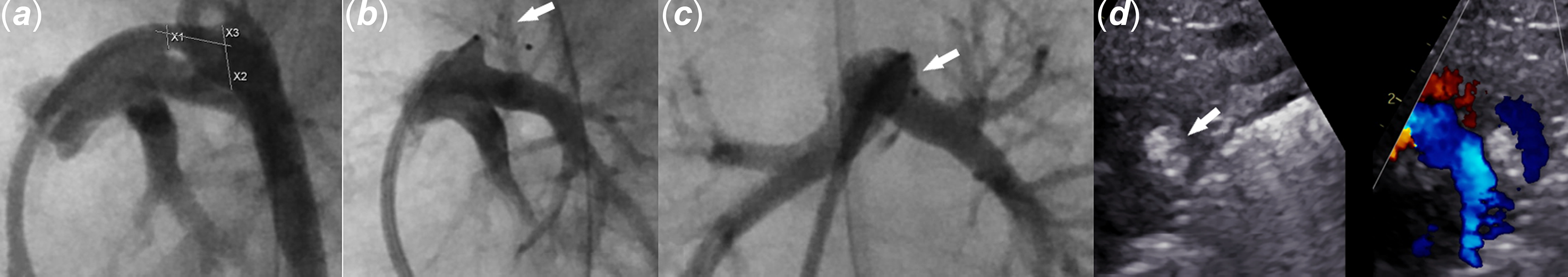
Figure 2. Images demonstrate the utility of intraprocedural angiogram and echocardiogram. A. Patent ductus arteriosus angiogram in the lateral projection with caudal angulation depicts measuring patent ductus arteriosus’s dimensions. B and C . Main pulmonary artery angiogram after KA micro plug deployment shows the device inside the patent ductus arteriosus (white arrow) with no left pulmonary artery obstruction. B . Lateral projection with caudal angulation. C . Frontal projection with left anterior oblique angulation. D. Still echocardiogram image shows the KA micro plug (arrow) inside the patent ductus arteriosus without residual shunt.
The 2.9-Fr 125 cm microcatheter included in the device package is cut to a length 12 cm longer than the length of the Angled Glide catheter (marked earlier on the back table drape), as seen in Figure 1B. This is achieved by placing the hub of the microcatheter at one of the marks on the back table’s drape. Then, the microcatheter is cut 12 cm longer than the second mark. This length was chosen to allow the microcatheter to pass the tip of the Angled Glide catheter for 2 cm (approximately 10 cm is needed to account for the length of the Y-connector, which will be connected to the Angled Glide catheter and the bulky back end of the microcatheter). Shortening the microcatheter serves two purposes: first, it allows easier control of the Angled Glide catheter and the microcatheter with the left hand during device deployment. Second, the microcatheter can be pulled back over the delivery wire to obtain an angiogram through the Angled Glide catheter before releasing the device. The first “Y-connector” is attached to the cut microcatheter and flushed. Then, the device is front-loaded and de-aired through the microcatheter inside a heparinised saline bowl (Figure 1C and Video 1). The device is kept just inside the distal end of the microcatheter.
The second “Y-connector” is attached to the Angled Glide catheter and de-aired. Then, the combination of the device and the cut microcatheter is advanced inside the Angled Glide catheter towards the tip. The Angled Glide catheter is pulled back over the microcatheter to expose the microcatheter (Figure 1D). The operator moves the two catheters together and pulls them back as one unit so the tip of the microcatheter is inside the ductus arteriosus. Then, the device is deployed inside the patent ductus arteriosus by “uncovering” the device. This “uncovering” manoeuvre is achieved by fixing the delivery wire with the right hand and pulling both catheters as one unit with the left hand over the delivery wire (Figure 1E and F and Video 2). When the ductus ampulla is larger than the selected device diameter, which is usually seen with a type A (conical) ductus, the authors sometimes deploy the first disc in the descending aorta close to the ampulla and slowly pull back the first disc inside the ampulla. Then, deploy the rest of the device inside the ductus arteriosus. After deployment, the microcatheter is pulled back over the delivery wire to a point just outside the angled glide catheter. The Y-connector attached to the Angled Glide catheter is tightened, and an angiogram is obtained in the main pulmonary artery (Figure 2 B and C). If the device position is unsatisfactory, the microcatheter is advanced over the delivery wire inside the Angled Glide catheter and used to recapture and redeploy the device. After confirming good positioning angiographically, an echocardiogram is obtained to assess the adequacy of closure, device position, and flow in the aortic arch and left pulmonary artery. Then, the device is released by performing the classic counterclockwise rotation on the delivery wire. The delivery wire and catheters are removed. Echocardiogram images are obtained again (Figure 2D). If the device needs to be removed after releasing it from the delivery wire, it can be snared out through the same 4-Fr Angled Glide catheter without the need to exchange the existing sheath for a longer or larger one. Lastly, the sheath is removed, and manual pressure is held until haemostasis is achieved.
Results
During the study period, 33 patients underwent attempted ductus arteriosus closure using the KA micro plug device. Fifteen patients were excluded as the weight at the procedure was >1500 g. All excluded patients underwent successful closure with no procedural-related complications.
Eighteen patients were included in the statistical analysis with a male-to-female 7 (39%): 11 (61%). The procedure was successful in all patients. The median weight, age, and corrected gestation age at the procedure were 943 g (682–1225), 26 days (9–79), and 28.5 weeks (25.6–32), respectively (Table 1).
Table 1. Summarising demographics
Fourteen patients (77.7%) had an F-type (fetal) ductus. Three patients (16.7%) had an A-type (conical). One patient (5.6%) had a C-type (tubular). The median narrowest ductus diameter was 1.8 mm (0.9–2.9) (Table 2).
Table 2. Summarising patent ductus arteriosus dimensions and types
The median procedure and fluoroscopy times were 28.5 min (18–53) and 4.6 min (2.2–9.4), respectively. The median radiation doses expressed as dose area product and air kerma were 1.81 µGy m2 (0.49–3.23) and 1.28 mGy (0.41–2.74), respectively. Six (33.3%) patients had minor haemodynamic instability requiring calcium gluconate administration in 5 (28%) patients and epinephrine administration in 1 (0.6%) patient. When comparing the subgroups in terms of haemodynamic instability, no difference was observed in the size of the ductus or the patients. All patients underwent closure while being on their high-frequency jet ventilators, as this is our preferred ventilation mode to minimise barotrauma. Reference Shibbani, Mohammad Nijres and McLennan13 None of the patients developed major haemodynamic instability.
All patients had immediate complete occlusion observed in the intraprocedural echocardiogram, except one patient in whom complete occlusion was seen in one-hour post-procedural echocardiogram. None of the procedures were complicated with iatrogenic left pulmonary artery or aortic flow obstruction. No catheter-induced tricuspid valve regurgitation was observed. Device embolisation or malposition was not encountered. In only 2 (11%) patients, more than one device was needed, and this primarily occurred early in our experience, within the first two months. For the first patient with an F-type ductus, with the narrowest ductus dimension of 2 mm, an initial 3 mm device was utilised, resulting in a significant residual shunt. The device was removed, and a 4 mm device was successfully deployed. In hindsight, the initial device selection was considered inappropriate (too small) for the ductus arteriosus size. In the second case with an A-type ductus, where the narrowest ductus diameter was 2.2 mm, a 4 mm device was initially selected but pulled through to the main pulmonary artery during deployment. The device was upsized to 5 mm, leading to successful closure. In retrospect, the initial device selection seemed appropriate, and probably excessive tension was applied during deployment causing the device to pull through the ductus arteriosus. In both cases, the first device was removed while still attached to the delivery wire. Since April 2022, after surpassing the learning curve, none of the patients required the use of more than one device.
The final placed devices were 1.2–2 mm larger than the smallest ductus arteriosus in all patients, except two patients. The first patient had an F-type ductus with the smallest diameter of 2.6 mm. The operator placed a 5 mm KA device with no complications. In hindsight, we believe a 4 mm device would have worked equally well. The second patient with an A-type ductus had the smallest ductus diameter of 2.2 mm. Initially, a 4 mm KA device was attempted, then upsized to a 5 mm KA micro plug as described earlier.
On a median follow-up of 10.9 months (0.1–19), all patients are alive with the device in position. No significant tricuspid valve regurgitation, late left pulmonary artery, or aortic arch obstruction was encountered.
Discussion
The findings of this study support those from San Diego. Reference Heyden, El-Said, Moore, Guyon, Katheria and Ratnayaka15,Reference Guyon, Duster and Katheria16 In this cohort of infants weighing less than 1500 g, the KA micro plug demonstrates effectiveness and safety in closing the patent ductus arteriosus. The procedure was successful in all patients, without major haemodynamic instability. No immediate iatrogenic left pulmonary artery, aortic obstruction, or tricuspid valve regurgitation was encountered. The incidence of using more than one device (11%) in this cohort is consistent with similar studies, showing an incidence of 8–17%. Reference Guyon, Duster and Katheria16,Reference Barry, Gudausky and Balzer20 During the study period, similar to the San Diego experience, we did not encounter late left pulmonary artery or aortic arch obstruction, which were rarely described with other devices. Reference Heyden, El-Said, Moore, Guyon, Katheria and Ratnayaka15,Reference Guyon, Duster and Katheria16,Reference Dalby, Shibbani and Mercadante21–Reference Markush, Tsing and Gupta24
The KA micro plug and the Piccolo device share many favourable features for transcatheter ductus arteriosus closure in premature infants. Both are soft and easy to visualise by fluoroscopy and echocardiogram and have radiopaque bands to enhance visualisation. Also, the two devices are available in short lengths and the procedure can be performed in antegrade fashion through a single small (4-Fr) sheath placed in the femoral vein. Both devices, though, share the drawback of being unable to be deployed with the sole use of a 4-Fr Angled Glide catheter, which is the most common catheter used to cross the tricuspid valve and access the ductus arteriosus. Reference Shibbani, Mohammad Nijres and McLennan13,Reference Heyden, El-Said, Moore, Guyon, Katheria and Ratnayaka15,Reference Guyon, Duster and Katheria16,Reference Sathanandam, Gutfinger and Morray25
The KA micro plug and Piccolo devices differ in a few aspects, as well. The delivery wire of the KA micro plug is thinner, contributing to the overall softness of the delivery system and theoretically reducing the incidence of haemodynamic instability. Also, the smaller profile of the KA micro plug prevents the device from being advanced easily through a 4-Fr Angled Glide catheter, necessitating the use of the 2.9-Fr microcatheter inside the Angled Glide catheter. On the other hand, the Piccolo device necessitates using the 4-Fr TorqVue LP catheter, which is stiffer and has a slightly larger inner diameter than the Angled Glide catheter (0.046” vs. 0.0405”). It is worth mentioning that the TorqVue LP catheter is 0.011” larger than the 0.035” Tiger wire, necessitating careful advancement to avoid vascular injuries from the wire-catheter mismatch. On the other hand, the mismatch is minimal between the 4-Fr Angled Glide catheter and the 0.035” Tiger wire. Reference Shibbani, Mohammad Nijres and McLennan13,Reference Heyden, El-Said, Moore, Guyon, Katheria and Ratnayaka15,Reference Guyon, Duster and Katheria16,Reference Rahde Bischoff, Backes, Weisz, Mohammad Nijres, McNamara and Kluckow19,Reference Sathanandam, Gutfinger and Morray25
There are also important size differences between the two devices. First, the KA micro plug is available in only one length (2.5 mm), with the dimension of the middle disc the same as the proximal and distal ones. The Piccolo is available in 3 different lengths (2,4, and 6 mm), with the middle disc having a smaller diameter and greater length than the other two discs. The Piccolo device is sized based on the diameter of the middle disc. For instance, the 3 mm Piccolo device has a middle disc of 3 mm, while the inner and the outer discs measure 4 mm. Reference Heyden, El-Said, Moore, Guyon, Katheria and Ratnayaka15,Reference Sathanandam, Gutfinger and O’Brien17,Reference Rahde Bischoff, Backes, Weisz, Mohammad Nijres, McNamara and Kluckow19 Therefore, the smallest KA device (3 mm device) is 1 mm smaller than the smallest 3 mm Piccolo device as described earlier. Likewise, the 6 mm KA micro plug device is smaller than the 5 mm Piccolo device. The proximal and distal discs of the 5 mm Piccolo device measure 6.5 mm.
Among the three common devices utilised for closure in premature infants (Piccolo, KA micro plug, and microvascular plug), the authors favour using the Piccolo device as it was designed, tested, and approved by the Food and Drug Administration for use in premature infants. Reference Sathanandam, Gutfinger and O’Brien17–Reference Barry, Gudausky and Balzer20 However, sometimes the authors elect to use the KA micro plug for smaller infants to avoid the need for exchanging for the stiff TorqVue LP catheter, especially when the intention is to place the smallest possible device.
Although some centres still employ the microvascular plug, it is not our preferred choice. This is mainly due to its long length and the challenge of visualising it by fluoroscopy, making the entire intraductal placement a challenging task. Reference Guyon, Duster and Katheria16,Reference Barry, Gudausky and Balzer20
We would like to offer some technical considerations which we believe are crucial when using the KA micro plug. First, cut the microcatheter to an appropriate length. If the microcatheter is shorter than the Angled Glide catheter, recapture of the device becomes challenging, and resistance will be encountered to push the device inside the Angled Glide catheter. Conversely, cutting the microcatheter too long can make controlling both catheters difficult for a single operator during deployment. Fixing both catheters with the left hand is challenging if the microcatheter is excessively long. If the operator is not planning to obtain a main pulmonary artery angiogram after deploying the device, cutting the microcatheter is unnecessary. However, controlling the Angled Glide catheter and microcatheter with a single operator becomes challenging due to the excessive length of the microcatheter.
Although cardiovascular injury was not encountered, caution should be practiced as placing the device inside the microcatheter stiffens it, and cutting the microcatheter could make the tip sharper. Pushing the microcatheter outside the Angled Glide catheter should be avoided to mitigate the risk of injury. Instead of pushing, uncover the microcatheter by pulling the Angled Glide catheter over the microcatheter. Then, the microcatheter is slowly brought back inside the ductus arteriosus. Furthermore, deploy the device by similarly uncovering it rather than pushing it out. The alternative technique involves cutting the microcatheter from the back end, as described by the San Diego group. Reference Heyden, El-Said, Moore, Guyon, Katheria and Ratnayaka15,Reference Guyon, Duster and Katheria16 However, this method does not allow for the attachment of the Y-connector to the catheter and may impact the adequacy of de-airing the device when loaded inside the cut microcatheter. In our technique, attaching two Y-connectors (one to the microcatheter and the other to the Angled Glide catheter) allows for excellent de-airing, as demonstrated in Video 1, and mitigates the risk of blood loss.
It is worth mentioning that other possible approaches, which have not yet been described, involve exploring the feasibility of closure using the KA micro plug solely guided by echocardiography, with or without the need to modify the microcatheter. In our cohort, we observed low procedure time, fluoroscopy time, and radiation dose. Our technique in crossing the tricuspid valve and the ductus is different from what was described by the San Diego group who advised to use of a combination of an angled microcatheter and a soft 0.014” wire to cross the tricuspid valve and the ductus arteriosus aiming to mitigate the risk of iatrogenic tricuspid valve regurgitation. Reference Heyden, El-Said, Moore, Guyon, Katheria and Ratnayaka15,Reference Guyon, Duster and Katheria16 Instead, we opt for a combination of the Angled Glide catheter and a 0.035” wire, commonly used by many other interventionalists, to cross the tricuspid valve and ductus arteriosus, which simplifies the procedure and requires less equipment. Reference Shibbani, Mohammad Nijres and McLennan13,Reference Sathanandam, Gutfinger and O’Brien17,Reference Morray, Sathanandam and Forbes18 Notably, we did not encounter any cases of worsening tricuspid valve regurgitation. Furthermore, we de-air and front-load the KA micro plug inside the microcatheter on the back table to save time and simplify the procedure. In this way, the microcatheter and the device can be advanced simultaneously as one unit rather than sequentially. Reference Heyden, El-Said, Moore, Guyon, Katheria and Ratnayaka15
The authors believe that neither the Piccolo nor the KA micro plug are ideal devices, and enhancements are needed. For the Piccolo device, we believe the TorqVue LP delivery catheter should be shorter than currently manufactured and be even softer to facilitate control, reduce catheter-induced tricuspid valve regurgitation, and reduce the tension on the ductus arteriosus during deployment. Additionally, the distal curve of these delivery catheters should be adjusted to allow the catheters to be used for both crossing and deploying the device, thus eliminating the need for catheter exchange. Furthermore, we recommend modifying the delivery wire to be less stiff by reducing its diameter or changing the material. The KA micro plug could become an ideal device with modifications that allow deploying and recapturing (if needed) through the same crossing catheter, eliminating the need for a cut microcatheter.
The study is limited by being a retrospective single-centre study with a small sample size. It was not powered sufficiently to compare outcomes with other available devices. In a recent multicentre study, no significant difference in the short-term outcomes was observed when comparing the Piccolo, KA Micro Plug, and Microvascular Plug. Reference Dalby, Shibbani and Mercadante26 The KA device was selected based on infants weighing less than 1500 g, and larger devices are probably needed for bigger infants. Due to the study’s retrospective nature and the small sample size, it was difficult to discern the precise cause of any haemodynamic instability and parse out whether it was a side effect of anaesthesia or a result of crossing the tricuspid valve. However, the haemodynamic instabilities encountered in some patients in this cohort were minimal and didn’t hinder the procedure’s success.
Conclusion
This cohort suggests that utilising the KA micro plug for transcatheter patent ductus arteriosus closure in premature infants weighing less than 1500 g is both effective and safe without significant haemodynamic instability. The authors recommend the manufacturers of the KA micro plug and Piccolo devices consider making changes to enhance safety and procedural ease.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1047951124025629.
Financial support
None.
Competing interests
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees (the Institutional Review Board at the University of Iowa).







