SARS-CoV-2, the virus responsible for the novel coronavirus disease-2019 (COVID-19), has infected millions of individuals worldwide with approximately 1.7 million infections in the United States at the time of writing.1 A growing body of literature has described cardiac manifestations of this disease in adults, including myocarditis and arrhythmias,Reference Wang, Hu and Hu2,Reference Goyal, Choi and Pinheiro3 electrocardiographic changes including ST segment elevations, T wave inversions, and laboratory abnormalities such as elevated high-sensitivity troponin T and N-terminal pro-brain-type natriuretic peptide. Although the pathophysiology is unclear, these abnormalities are associated with increased morbidity, such as need for intubation, and mortality.Reference Bonow, Fonarow, O’Gara and Yancy4–Reference Tersalvi, Vicenzi, Calabretta, Biasco, Pedrazzini and Winterton8 Adults have been disproportionately affected, with children making up under 3% of the infected population.1,Reference Dong, Mo and Hu9 Given the smaller numbers of paediatric cases, there is a paucity of cardiac data available.
While recommendations have been published with regards to general paediatrics,Reference Carlotti, Carvalho, Johnston, Rodriguez and Delgado10 paediatric cardiac catheterisation,Reference Morray, Gordon and Crystal11 cardiothoracic surgery,Reference Ryan, Melendres-Groves and Zamanian12 and pulmonary hypertension,Reference Stephens, Dearani and Guleserian13,Reference Horn, Chakinala, Oudiz, Joseloff and Rosenzweig14 there remains a lack of consensus guidelines for non-interventional cardiac workup and monitoring of children hospitalised with COVID-19. In this report, we share our approach to the cardiac workup and monitoring of these children with regards to laboratory, electrocardiographic, and imaging evaluation of children with respiratory presentations of COVID-19 with the goal of increasing awareness of the cardiac manifestations of this disease, suggested diagnostic tests, and when to call the paediatric cardiologist. This was based on our experience at a major congenital heart centre in New York City, the epicentre of the COVID-19 pandemic in the United States. During this time, all paediatric admissions to NewYork-Presbyterian Hospital system sites across New York City were consolidated at Morgan Stanley Children’s Hospital. Recently, groups have reported children with COVID-19, or exposure to close contacts with COVID-19, who develop a multi-system inflammatory syndrome.Reference Jones, Mills and Suarez15,Reference Riphagen, Gomez, Gonzalez-Martinez, Wilkinson and Theocharis16 The protocols outlined here are intended for application to acute presentation of the “classic” respiratory presentation of COVID-19. Our institution is currently developing a separate multidisciplinary protocol detailing our approach to this new entity, which will be submitted for publication separately.
Criteria for admission at our institution include, but are not limited to, respiratory distress, supplemental oxygen requirement, cardiovascular instability, dehydration, or medications requiring inpatient monitoring. In deciding the appropriate workup, we consider suspicion for cardiac involvement and acuity level (Table 1).
Table 1. Cardiac workup and monitoring in children with and without suspicion for cardiac disease
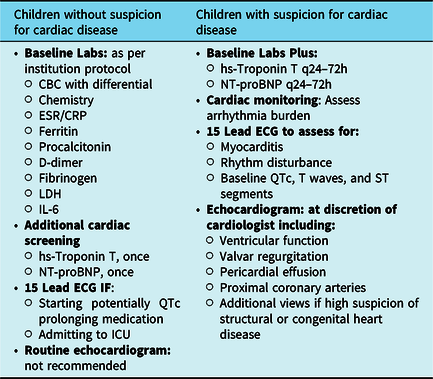
CBC = complete blood count; CRP = C-reactive protein; ECG = electrocardiogram; ESR = erythrocyte sedimentation rate; hs-Troponin T = high-sensitivity troponin T; IL-6 = interleukin 6; LDH = lactate dehydrogenase; NT-proBNP = N-terminal pro-brain-type natriuretic peptide
Children without suspicion for cardiac involvement
Patients with normal blood pressure and cardiac exam, absence of hepatosplenomegaly, normal cardiac size on chest X-ray, normal high-sensitivity troponin T and N-terminal pro-brain-type natriuretic peptide, and who do not meet criteria for multi-system inflammatory syndrome can be considered low risk for cardiac involvement. All children with confirmed or suspected COVID-19 should have basic laboratory studies drawn according to their institution specific protocol, but should include at least a complete blood count with differential, electrolytes, renal and liver function tests, erythrocyte sedimentation rate, and C-reactive protein. In addition, a screening high-sensitivity troponin T and N-terminal pro-brain-type natriuretic peptide should be measured in all admitted children. A chest X-ray is useful in assessing cardiac size, confirming clinically suspected pneumonia, and evaluating respiratory failure, and should be obtained for children admitted with COVID-19.
We do not obtain routine electrocardiograms or echocardiograms for children with acute respiratory presentations of COVID-19 without suspicion for cardiac disease given the added exposure to staff. However, an electrocardiogram should be obtained for patients requiring ICU admission, as well as prior to starting therapy with corrected QT interval (QTc) prolonging medications, such as hydroxychloroquine, azithromycin, and lopinavir/ritonavir,Reference Naksuk, Lazar and Peeraphatdid17 regardless of suspicion for cardiac involvement. Because the QTc appears to achieve peak prolongation between days 3 and 4 of treatmentReference Chorin, Dai and Shulman18 and hydroxychloroquine has a long half-life,19 these patients should continue to have their QTc monitored after completing the medication course. Due to a recent study showing increased rates of arrhythmia and mortality with hydroxychloroquine use,Reference Mehra, Desai, Ruschitzka and Patel20 its role in the treatment of children with severe COVID-19 is unclear. However, since the impact of other experimental therapies such as remdesivir is not yet known, we suggest close monitoring of the QTc even in the absence of hydroxychloroquine use.
In cases where hydroxychloroquine is still being used, published recommendations suggest that the QTc should be less than 500 ms for patients with normal QRS durations and less than 520 ms for patients with prolonged QRS durations prior to initiating therapy. If the QTc is borderline (460–500 ms), treatment may be initiated with caution.Reference Chorin, Dai and Shulman18,Reference Giudicessi, Noseworthy, Friedman and Ackerman21
More frequent monitoring of the QTc may be warranted in patients with comorbid conditions known to prolong the QTc, including congenital long QT syndrome, severe renal insufficiency,Reference Malhis, Al-Bitar, Farhood and Zaiat22 treatment with other QTc prolonging medications, and electrolyte disturbances such as hypokalaemia, hypomagnesemia, and hypocalcemia. Electrolyte abnormalities should be corrected prior to initiating QTc prolonging therapies and electrolytes should be closely monitored for the duration of therapy. Concurrent use of other QTc prolonging medications should be avoided.
Children with suspicion or at risk for cardiac involvement
Growing evidence suggests common involvement of multiple organ systems including the heart, kidneys, and brain.Reference Sharifi-Razavi, Karimi and Rouhani23–Reference Lai, Shih, Ko, Tang and Hsueh26 There are many situations for which the paediatric cardiology team might be consulted. These may include abnormal physical exam findings suggestive of heart failure, such as inappropriate tachycardia or arrhythmia, gallop, pericardial friction rub, or jugular venous distention, evidence of cardiogenic shock, or pulmonary hypertension. Elevated high-sensitivity troponin T or N-terminal pro-brain-type natriuretic peptide or cardiomegaly on chest X-ray also warrant a cardiology consult. The paediatric cardiologist may also be consulted for abnormal electrocardiogram, including changes associated with pericardial or myocardial inflammation.
Patients who are at risk for severe infection and may be more likely to require admission to the ICU include infants and those with underlying comorbidities such as lung disease, cardiovascular disease, chronic kidney disease, diabetes, and metabolic or immune disorders.Reference Shen, Yang and Jiang27,Reference Yang, Zheng and Gou28 Based on consensus opinion at our institution, we suggest that special consideration should be given to patients with pulmonary hypertension, unrepaired complex congenital heart disease, cyanotic heart disease with oxygen saturations less than 85%, ventricular dysfunction requiring medical therapy, and history of heart transplantation on immunosuppressive medications. Due to their higher risk profile, these patients may warrant a more complete evaluation than those without pre-existing conditions.
Laboratory studies
In accordance with institutional COVID-19 protocol, children with confirmed or suspected acute COVID-19 requiring admission undergo laboratory workup including complete blood count, chemistry, inflammatory markers, high-sensitivity troponin T, and N-terminal pro-brain-type natriuretic peptide. In our experience, for children with suspected cardiac involvement, it is useful to trend high-sensitivity troponin T and N-terminal pro-brain-type natriuretic peptide every 24–72 hours. If these are grossly abnormal or continuing to rise, we obtain an electrocardiogram and echocardiogram and consider transfer to the ICU, if not already admitted there.
Electrocardiography/reviewable cardiac monitoring
Children with suspicion for cardiac involvement or multi-system inflammatory syndrome should have a screening electrocardiogram to assess for myocarditis or arrhythmia and establish a baseline for QTcs, T waves, and ST segments. If the electrocardiogram has findings suggestive of ischaemia or inflammation, such as T-wave inversions or ST segment elevations or depressions, we suggest obtaining electrocardiograms daily until the abnormalities resolve or the clinical situation has improved. All patients with suspicion for cardiac involvement, as well as those admitted to the ICU, should be placed on reviewable cardiac monitoring to assess arrhythmia burden.
Echocardiography
The decision to perform an echocardiogram is made at the discretion of the paediatric cardiologist based on clinical, laboratory, and electrocardiographic data, with careful consideration given to reducing viral exposure for staff in the setting of widespread infection. At our institution, we developed a protocol for focused studies, keeping in mind the following goals such as assess for signs of myocarditis or myocardial dysfunction, evaluate for evidence of pulmonary hypertension/pulmonary embolus, and minimise exposure to the sonographer. We suggest obtaining an echocardiogram for those children with suspicion for cardiac involvement, including those with physical examination findings concerning for heart failure, abnormal laboratory values or electrocardiograms with evidence of myocardial injury, or who present with multi-system inflammatory syndrome. Our current protocol includes assessment of ventricular function, valvar regurgitation, presence of pericardial effusion, and, with the newly described multi-system inflammatory syndrome in childrenReference Jones, Mills and Suarez15,Reference Riphagen, Gomez, Gonzalez-Martinez, Wilkinson and Theocharis16 in mind, evaluation of the proximal coronary arteries in children with confirmed or suspected COVID-19 who require an echocardiogram. Children with known or suspected congenital heart disease should have a complete congenital transthoracic echocardiogram performed when necessary. In order to limit staff exposure, all measurements are done after the sonographer has left the patient room.
Although extracorporeal membrane oxygenation use appears to be infrequent in children with COVID-19,Reference Shekerdemian, Mahmood and Wolfe29 there is evidence that it may provide a survival benefit for adults with severe infection,Reference Jacobs, Stammers and St Louis30 though its use is not consistent across all centres.Reference Cummings, Baldwin and Abrams31 In this situation, echocardiography may be required to assess cannula position, ventricular function, and presence of left atrial hypertension.
In our opinion, following hospital discharge, a complete follow-up echocardiogram is recommended in the out-patient setting for those children with abnormalities on the initial study. The optimal timing of follow-up has yet to be established and will be determined primarily by considering the clinical context as well as staff exposure and facility capacity in the setting of the evolving pandemic.
Arrhythmia
Arrhythmia has been reported as a significant issue in adult patients with COVID-19Reference Wang, Hu and Hu2,Reference Goyal, Choi and Pinheiro3 and may be secondary to myocardial injury or direct effects of cytokines on sodium and potassium channels.Reference Lazzerini, Boutjdir and Capecchi32 Interleukin-6 has been shown to be elevated in those with myocardial injury compared with those without.Reference Chen, Zhou and Wang33 These same cytokines may also inhibit enzymes required for clearing certain QTc prolonging medications, thereby enhancing their impact on the QTc.Reference Cummings, Baldwin and Abrams31 Other potential etiologies for arrhythmia include metabolic disturbance,Reference Chen, Guo and Sun34 hypoxia,Reference Clark, Christlieb, Sanmarco, Diaz-Perez and Dammann35 and genetic dysrhythmias, which may be revealed by acute illness or significant stressors, such as Brugada syndromeReference Rattanawong, Vutthikraivit and Charoensri36 and long QT syndromes.Reference Shwartz, Crotti and Insolia37 If there is an associated elevation in high-sensitivity troponin T, early myocarditis should be considered.Reference Smith, Ladenson, Mason and Jaffe38 Due to the risk of arrhythmia, we suggest that these patients be placed on reviewable cardiac monitoring.
Limitations
Significant limitations exist that may impact the relevance of this approach, most importantly that the practices outlined in this report are based primarily on expert opinion and adult literature rather than paediatric data. The number of children with COVID-19 worldwide is small relative to the number of adults and our understanding of it is evolving rapidly, resulting in relatively limited data addressing paediatric cardiac involvement. Prospective data collection is necessarily impeded by the need to minimise exposure of phlebotomy, electrocardiogram, and echocardiogram staff. Furthermore, the natural history and underlying pathophysiology of this disease in children, including the newly emerging multi-system inflammatory syndrome in children, is just now being described. These practices reflect our best understanding at the time of writing; however, our knowledge of this disease and its various presentations is constantly evolving and clinical practice must necessarily evolve in-kind.
Conclusions
Providers caring for children with acute COVID-19 should keep cardiac involvement in mind and consult paediatric cardiology when there is suspicion for cardiac injury. In our opinion, the extent of workup and monitoring of these patients should be based on careful determination of clinical suspicion for cardiac disease and level of acuity of each patient, as well as the expected impact the information will have on clinical management. As the full picture of the multi-system inflammatory syndrome in children emerges, the role of the paediatric cardiologist will likely evolve and indications for testing may change. More information obtained from multi-centre collaboration is needed to establish definitive guidelines.
Acknowledgements
The authors acknowledge the contributions of the following members of the Columbia University Irving Medical Center Pediatric-Adult Congenital Heart Research Collaborative: Emile Bacha, Eva Cheung, Kanwal Farooqi, David Kalfa, Ganga Krishnamurthy, Matthew Lewis, Damien LaPar, Marlon Rosenbaum, and Julie Vincent.
Financial Support
Dr Anderson receives salary support from the National Institutes of Health/NHLBI (K23 HL133454).
Conflicts of Interest
None.



