Introduction
There is a rich history of research on the chemical ecology of forest insects conducted by forest entomologists and chemical ecologists in Canada. Canadian researchers have been involved in the identification (Table 1), development, and implementation of semiochemical-based pest management as part of sustainable forest management in Canada (Fig. 1). Forest insect pests are good targets for management with semiochemicals because forest pest management is often focussed on a key pest at a given time in a given location. This makes the use of species-specific signals a feasible option that might not be realised in agricultural or urban settings where mangers frequently deal with pest complexes (Borden Reference Borden1993). Semiochemicals are an integral part of integrated pest management (IPM) of insects considered to be forestry pests in Canada. This review will highlight the efforts of Canadian researchers in the implementation of semiochemical-based tools into various aspects of IPM of forest insect pests (Fig. 1). Although the research conducted in Canada has been adopted in other countries around the world, the focus of this review is semiochemical-based management of forest insects in Canada that has been implemented as a result of Canadian research.
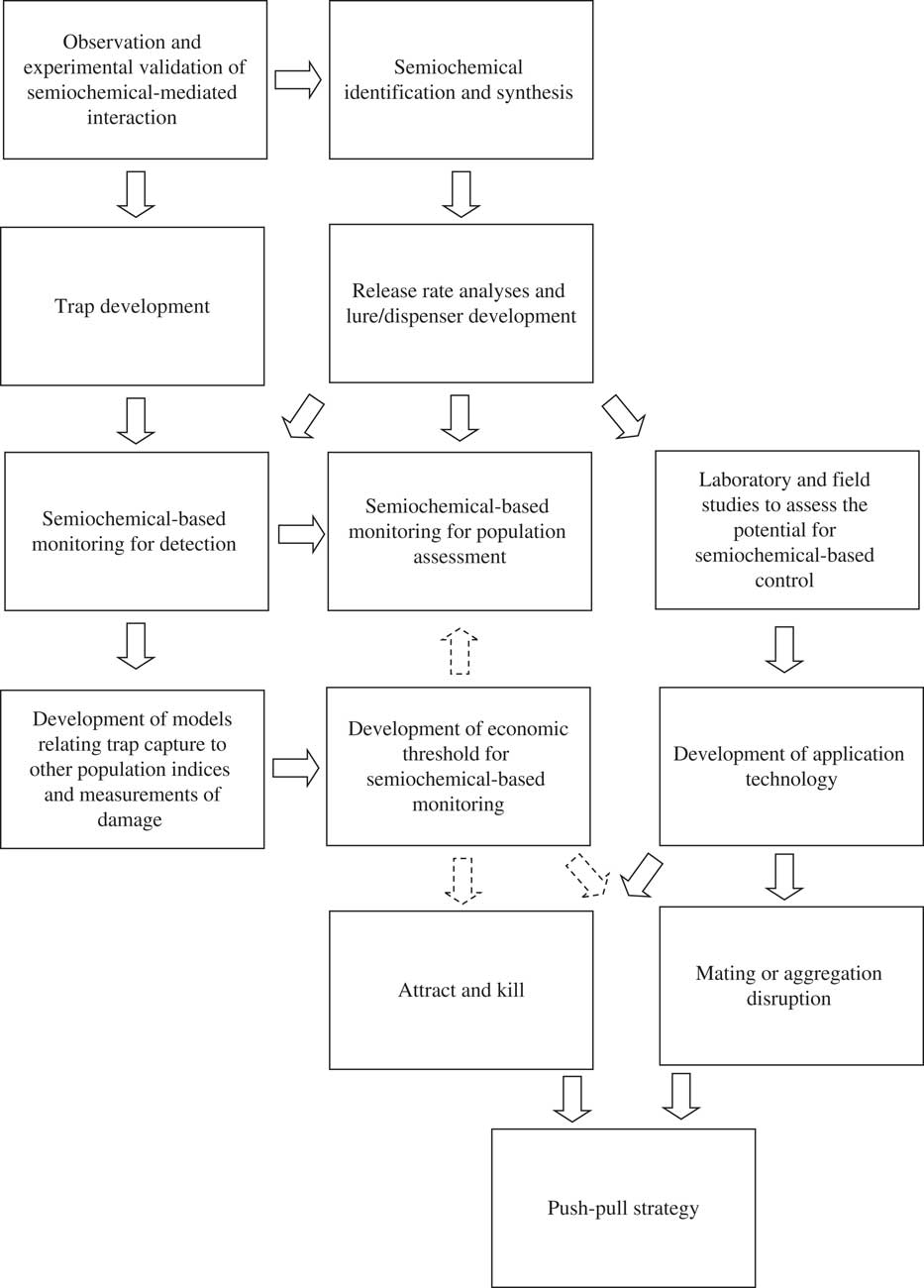
Fig. 1 Research and development required to implement semiochemicals into IPM programmes. Solid arrows indicate flow of information. Dashed arrows indicate information that could be used to refine the semiochemical-based tactic.
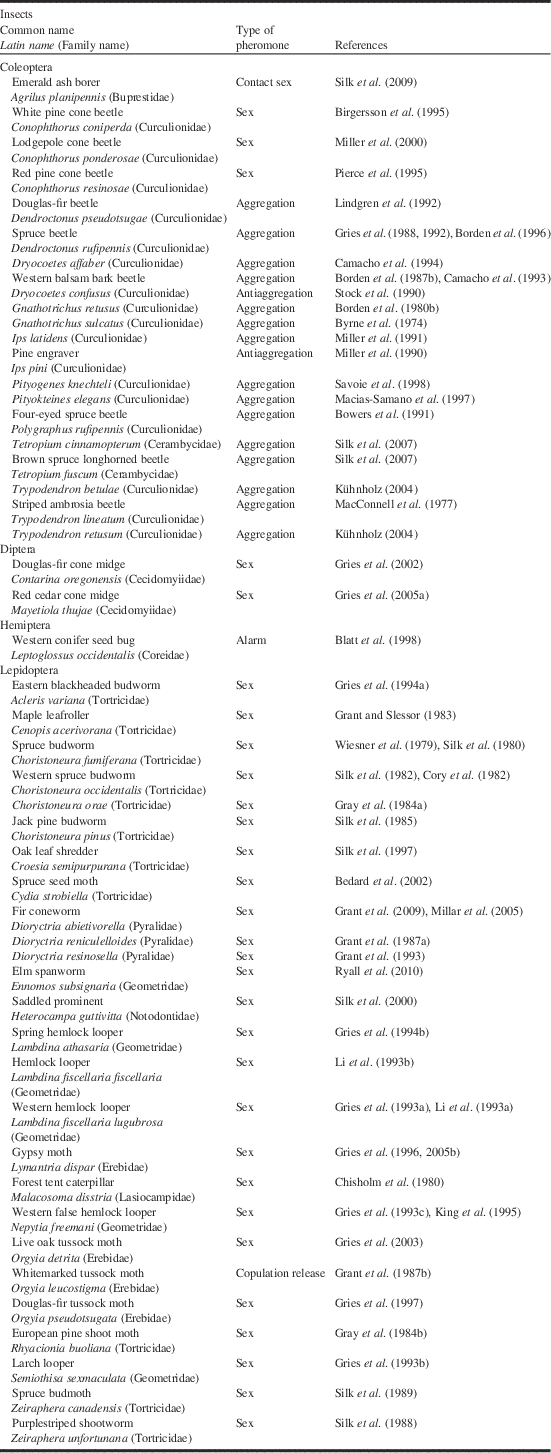
Table 1 Identification and/or field testing of pheromones of Canadian forest pests conducted in Canada.
What are semiochemicals?
Most insects use message-bearing chemicals, or semiochemicals (Nordlund and Lewis Reference Nordlund and Lewis1976), to facilitate important behaviours such as mating, oviposition, and foraging for resources. Semiochemicals have been classified into several functional categories based on the type of signal they communicate and the relationship between the receiver and the signaller in the communication channel. Pheromones are semiochemicals that are species-specific signals used for intraspecific communication. Pheromones can mediate mate location, courtship, aggregation, alarm, and spacing of insects throughout a resource. Sex pheromones that are released by one sex and evoke a response in the other sex for the purposes of mate location and aggregation pheromones that are released by one sex and evoke a response in both sexes are the most common pheromones exploited in IPM.
Allelochemics are semiochemicals that are emitted by individuals of one species and perceived by individuals of a different species (Whittaker and Feeny Reference Whittaker and Feeny1971). In many instances, allelochemics have evolved as signals for intraspecific communication, which are subsequently exploited as reliable signals by other organisms (Haynes and Yeargan Reference Haynes and Yeargan1999). Allelochemics are further categorised by the type of interspecific interaction that they mediate. Kairomones mediate an interaction that benefits the receiver of the signal, such as orientation of predaceous checkered beetles (Coleoptera: Cleridae) to the aggregation pheromones of their bark beetle prey (Coleoptera: Curculionidae: Scolytinae) (Poland and Borden Reference Poland and Borden1997). Allomones are semiochemicals that mediate an interaction that is beneficial to the emitter of the signal. Aggressive chemical mimicry employing allomones occurs in arthropods, whereby the signalling species mimics a chemical signal that is similar to that used for intraspecific communication in the receiving species (Haynes and Yeargan Reference Haynes and Yeargan1999). The receiver is “duped” into responding to the allomone and often becomes the prey item of the signaller. Synomones are semiochemicals that mediate an interspecific interaction that is beneficial to both the emitter and receiver of the signal. Herbivore-induced plant volatiles act as synomones that recruit natural enemies of the herbivore to the affected plant (Turlings et al. Reference Turlings, Tumlinson and Lewis1990). In mate-finding communication, synomones act to reduce competition in the chemical communication channel among closely related species with overlapping pheromone components (Evenden et al. Reference Evenden, Judd and Borden1999) to prevent wasted time and energy in orientation to heterospecifics.
The reliance of insects on semiochemicals for reproduction and survival makes them good targets for use in IPM. Various stages of research and development (Fig. 1) need to occur before semiochemicals can be incorporated into IPM programmes.
Monitoring pests with semiochemicals
Insect semiochemicals can be used in IPM for both monitoring and direct control of pest populations (Gut et al. Reference Gut, Stelinski, Thomson and Miller2004; Baker and Heath Reference Baker and Heath2005; Witzgall et al. Reference Witzgall, Cork and Kirsch2010). The most common commercial application of semiochemicals in pest management is for monitoring pest populations (Witzgall et al. Reference Witzgall, Cork and Kirsch2010), and the majority of these products use synthetic copies of species-specific pheromone signals as lures in traps. There are several advantages to using pheromones to monitor pest populations: (1) signals are species specific and usually few non-target species are captured; (2) signals can attract insects from a distance and monitor low pest population densities; and (3) only small quantities of the active ingredient are necessary to elicit a response (Witzgall et al. Reference Witzgall, Cork and Kirsch2010). Several pest management strategies employ pheromone-baited traps to monitor populations for different purposes.
In the simplest way, pheromone-based monitoring can determine the presence of a given insect species in a forest ecosystem. Because pheromone-baited traps are more sensitive than many other sampling tools, this approach is particularly useful to detect the introduction and spread of invasive species on a landscape (Sweeney et al. Reference Sweeney, de Groot, Price and Gutowski2006). This approach has been widely used in Canada to monitor the introduction and spread of gypsy moth, Lymantria dispar (Linnaeus) (Lepidoptera: Erebidae) (Nealis Reference Nealis2009). Beyond detection of the presence of an insect, capture of the target insect over time can provide information on the diel periodicity of pheromone response (Shepherd Reference Shepherd1979) and the seasonal flight period (Grant et al. Reference Grant, de Groot, Langevin, Katovich, Slessor and Miller2002; Rocchini et al. Reference Rocchini, Bennett and Lindgren2003) and, in combination with phenological models, can be used to help time control measures (Régnière and Nealis Reference Régnière and Nealis2002). Trap catch in pheromone-baited traps was used to develop a degree-day model, which is used to predict adult emergence in the pine shoot moth, Rhyaciona buoliana (Denis and Schiffermüller) (Lepidoptera: Tortricidae) in order to time the application of control sprays in lodgepole pine seed orchards in British Columbia, Canada (Heeley et al. Reference Heeley, Alfaro, Humble and Strong2003).
Additional information about the population can be garnered from closer examination of the trapped insects, such as sex ratio (Borden et al. Reference Borden, Lafontaine and Pureswaran2008), mating status (Bergh et al. Reference Bergh, Seabrook and Eveleigh1988), size and disease status (Sweeney and McLean Reference Sweeney and McLean1987; Jones and Evenden Reference Jones and Evenden2008). These factors can be useful in determining the population phase of forest insects that undergo cyclical changes in population density. Pheromone-baited traps can also be used to measure genetic diversity of captured insects, which can be used to trace the path of invasion in introduced species (Carter et al. Reference Carter, Smith, Turgeon and Harrison2009). Pheromone response, as a phenotypic trait, can be used to delineate species within closely related species complexes or to uncover otherwise cryptic species (Sanders et al. Reference Sanders, Ennis and Daterman1977).
Pheromone-baited traps are less often developed to predict pest population densities and subsequent damage. This is because the relationship between trap catches and resulting damage is costly to develop and not very precise. In well-studied cases, economic thresholds, based on the numbers of insects captured in pheromone-baited traps, can be developed to trigger management action (Fig. 1) (Grant Reference Grant1991). Years of research led to the development of an operational pheromone-based monitoring system for the spruce budworm, Choristoneura fumiferana (Clemens) (Lepidoptera: Tortricidae) that can predict densities of larvae in the subsequent generation (Sanders Reference Sanders1988). In monitoring programmes for Douglas-fir tussock moth, Orgyia pseudotsugata (McDunnough) (Lepidoptera: Erebidae), pheromone trap capture combined with egg-mass surveys predict population concentrations and potential defoliation in Douglas-fir stands (Shepherd et al. Reference Shepherd, Daterman, Chorney and Gray1985). Pheromone-based monitoring is particularly useful for monitoring forest insect pests that go through cyclical changes in population density in which insect abundance can increase quickly and result in widespread damage.
Control of pests with semiochemicals
Semiochemicals can also be used to manipulate insect behaviour to interfere with reproduction and/or survival in order to control the pest population. Disruption of pheromone-mediated mating or aggregation behaviour is a pest management tactic that is achieved differently for different species. Mating disruption is normally achieved through the release of large amounts of synthetic sex pheromone to the managed area in an effort to disrupt mate-finding behaviour through a variety of mechanisms (Bartell Reference Bartell1982). This tactic has been best studied as a potential control for lepidopteran pests (Witzgall et al. Reference Witzgall, Cork and Kirsch2010). The recent registration of a sprayable pheromone formulation to target the spruce budworm (Rhainds et al. Reference Rhainds, Kettela and Silk2012) in Canada suggests that commercial application of pheromone-based mating disruption can now be added to the IPM tactics used against this important forest defoliator. Disruption of semiochemical-mediated aggregation behaviour has been attempted for tree-killing bark beetles (Coleoptera: Curculionidae: Scolytinae). Recent studies focussed on the use of antiaggregation pheromones (Borden et al. Reference Borden, Sparrow and Gervan2007) and non-host plant volatiles (Huber and Borden Reference Huber and Borden2001a) to interrupt mate finding and host location behaviours in an effort to save high-value trees from colonisation (Borden et al. Reference Borden, Sparrow and Gervan2007). Disruption of bark beetle orientation away from host trees can be coupled with deployment of attractive semiochemicals in a “push-pull” tactic (Borden et al. Reference Borden, Burleigh and Birmingham2006). This method is used to repel beetles away from high-value stands and concentrate them in areas from which they can be easily removed.
Population control can also be achieved by attracting the target insect pest to a semiochemical lure and subsequent removal of the attracted pest from the population. This strategy can be achieved using “attract and kill” or mass-trapping tactics. Attract-and-kill formulations combine an attractant and a killing agent to kill the attracted pest. This approach is well suited to bark-beetle management because both male and female beetles respond to aggregation pheromones and tree host volatiles in the tree colonisation process (Conn et al. Reference Conn, Borden, Scott, Friskie, Pierce and Oehlschlager1983). Synthetic copies of these attractants can be used to bait trap logs that are coated with insecticide (Fuchs and Borden Reference Fuchs and Borden1985) or trap trees that are injected with insecticide (Maclauchlan et al. Reference Maclauchlan, Borden, D’Auria and Wheeler1988) or felled and stripped or burned after beetle attack (Borden Reference Borden1990). Perhaps because only males are attracted to lepidopteran sex pheromones, attract-and-kill tactics have been less widely developed for lepidopteran forest defoliators. Several formulations show promise for control of pests of managed seed orchards (Sukovota et al. Reference Sukovata, Kolk and CieSlak2004). In these cases, the formulations contain synthetic sex pheromone as the attractant and pyrethroid insecticides as the killing agent. Mass trapping is similar to attract and kill, except that the attracted insects are contained within a trap and are physically removed from the population. Due to the cost of deploying large numbers of traps into a forested ecosystem, mass trapping is restricted to use in high-value stands (Borden Reference Borden1993) or small, discrete managed areas such as dry-land sorts (Lindgren and Fraser Reference Lindgren and Fraser1994).
Goals of review
Earlier reviews have focussed on the potential to use semiochemicals to: (1) manage coniferous forest pests of North America (Borden Reference Borden1993); (2) control forest Lepidoptera in eastern Canada (Silk and Kuenen Reference Silk and Kuenen1984); (3) monitor forest defoliators in North America (Grant Reference Grant1991); or (4) mitigate aggregation behaviour of bark beetles (Borden Reference Borden1997). Other reviews have specifically targeted semiochemical management of key forest pests, such as the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Curculionidae) (Borden and Lindgren Reference Borden and Lindgren1989) or the spruce budworm (Rhainds et al. Reference Rhainds, Kettela and Silk2012). The goals of the current review are to: (1) highlight research conducted on semiochemical-based management of forest pests in Canada; (2) discuss the current and potential uses of semiochemicals in IPM of forest pests in Canada; and (3) evaluate potential areas for increased research and implementation of semiochemicals into the management of forest pests in Canada.
Use of semiochemicals in management of forest defoliators
Monitoring forest defoliators
Monitoring is the pest management strategy that most commonly incorporates the use of semiochemicals in IPM (Witzgall et al. Reference Witzgall, Cork and Kirsch2010). Semiochemical-based monitoring of forest defoliators uses mostly sex pheromone-baited traps to detect the presence of a species in a given area or to monitor population change over time. Monitoring programmes along this continuum have existed at the research to operational scales for at least 19 species of forest defoliators in Canada (Grant Reference Grant1991). Systematic pheromone-based monitoring of forest defoliators across Canada has become a multi-jurisdictional responsibility under the purview of the Canadian Council of Forest Ministers (Canadian Council of Forest Ministers 2012).
Monitoring for detection: The gypsy moth, an insect pest of over 300 hardwood tree species, was accidentally introduced into the United States of America in the late 1860s. Since then, its range has expanded in North America (Liebhold et al. Reference Liebhold, Luzader, Reardon, Roberts, Ravlin and Sharon1998) and it is now established in eastern but not western Canada. This insect is a native of both Asia and Europe, with apparently two races that differ in size, flight characteristics, and host preferences. The Asian race is much larger than the European race, feeding on over 500 tree species and, in addition, both sexes of the Asian race are strong fliers compared with only males of the European race (Humble and Stewart Reference Humble and Stewart1994). Until recently, most research efforts were focussed on the European race, but in 1991, the Asian gypsy moth was discovered in Vancouver, British Columbia, Canada and in Washington, Oregon, and Ohio states, United States of America (Humble and Stewart Reference Humble and Stewart1994). The European race is now established in Ontario, Québec, New Brunswick, and Nova Scotia, Canada but, despite repeated introductions, has not yet established in British Columbia (Nealis Reference Nealis2009). The Asian race is not established anywhere in Canada and its occasional detection through pheromone-based trapping immediately triggers eradication efforts.
Early identification of the pheromone of the gypsy moth as cis-7,8-epoxy-2-methyloctadecane or disparlure, followed by the discovery that only the (+)-disparlure enantiomer mediates attraction of male moths (Cardé et al. Reference Cardé, Doane, Baker, Iwaki and Marumo1977), paved the way for the use of pheromone-baited traps to detect and delimit populations of this insect. Delta traps baited with 500 µg of (+)-disparlure (Grant Reference Grant1991) are deployed annually across Canada. In British Columbia, where the European gypsy moth has been repeatedly introduced but has not established, pheromone-baited traps are deployed in a grid pattern at a density of one trap per 2.6 km2 (Nealis Reference Nealis2009) and play a key role in directing eradication efforts against this insect (Fig. 2). Positive moth capture triggers labour-intensive egg mass surveys, intensified trapping the following season and insecticidal control (Nealis Reference Nealis2009) (Fig. 2). Although different densities of traps on the landscape in different years make it difficult to compare population densities of gypsy moth over time, only detection is required to initiate actions to eradicate this insect in western Canada. Pheromone-baited traps are also used to assess the effectiveness of eradication efforts. Pheromone-baited traps are less useful in areas where gypsy moth is established, but are still used to delimit the edges of population growth in those regions (Nealis and Erb Reference Nealis and Erb1993).
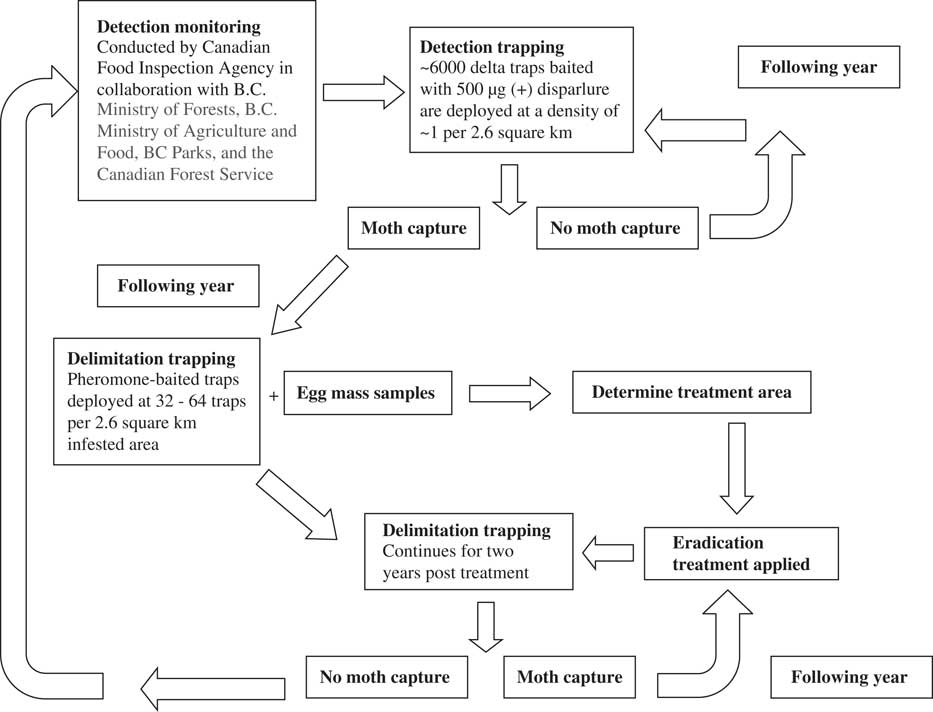
Fig. 2 Use of pheromone-baited traps for detection of gypsy moth (Lymantria dispar Linnaeus (Lepidoptera: Erebidae)) to inform eradication efforts of this insect in British Columbia (B.C.) based on information in (Nealis Reference Nealis2009).
Monitoring for population assessment: Management of native forest defoliators in Canada also relies on capture of male moths with pheromone-baited traps (Grant Reference Grant1991). Detection of population change of native forest defoliators that undergo cyclical changes in population density can assist forest managers and provide time for management response before populations reach outbreak densities. Providing a reliable population estimate can be obtained, pheromone-baited traps can be a cheaper and less labour-intensive means of population monitoring compared with sampling immature stages or aerial surveys of defoliation (Sanders Reference Sanders1988). This type of monitoring is fundamentally different from that used in detection, as moth capture in pheromone-baited traps needs to quantitatively reflect insect abundance. Consistency in sampling technique between years is required for monitoring of population assessment, and therefore development of an optimal trap and lure system is necessary before a monitoring system can be deployed (Grant Reference Grant1991).
After identification of the sex pheromone itself (Table 1), some of the most important factors that influence the efficacy of a pheromone-based monitoring system are the lure type (Sanders and Meighen Reference Sanders and Meighen1987), pheromone dose and the trap type (Evenden et al. Reference Evenden, Borden, Van Sickle and Gries1995b) and the trap spacing and density at each site (Houseweart et al. Reference Houseweart, Sanders and Jennings1981). Lure type and pheromone dose both can influence the active space (Byers Reference Byers2008) of the pheromone plume and dictate the distance from which moths can be attracted and the period over which the lure remains attractive. A pheromone dose that is consistently effective at different population densities (Sanders Reference Sanders1986a), but does not necessarily elicit the highest trap capture, should be used (Shepherd et al. Reference Shepherd, Daterman, Chorney and Gray1985). In fact, trap capture in low-dose pheromone-baited traps is easier to enumerate and can better reflect the actual population density than high-dose traps that capture more insects. Moths captured in traps baited with a low pheromone dose (10 µg) targeting the western hemlock looper, Lambdina fiscellaria lugubrosa Guenée (Lepidoptera: Geometridae) predicted egg counts in the subsequent generation better than traps baited with higher doses (Evenden et al. Reference Evenden, Borden and Van Sickle1995a). Similarly, moth capture in traps baited with a moderate pheromone dose of 0.01% pheromone by weight in polyvinyl chloride (PVC) rods best reflected population trends at various densities of the Douglas-fir tussock moth (Shepherd et al. Reference Shepherd, Daterman, Chorney and Gray1985). The operational pheromone-monitoring programme for spruce budworm was developed using PVC pellets loaded with 0.03% synthetic pheromone (Sanders Reference Sanders1981, Reference Sanders1988). The attractiveness of PVC pellets declined over time during the six-week trapping period and showed variation between batches of lures (Sanders and Meighen Reference Sanders and Meighen1987). Further research (Silk and Kuenen Reference Silk and Kuenen1986; Sanders Reference Sanders1990) led to the development of rubber septa lures to provide a controlled release of spruce budworm pheromone suitable for season-long monitoring.
Early monitoring efforts relied on the use of sticky traps with a limited surface area for moth capture. Sticky traps are inexpensive but can quickly become saturated with insects as the population density increases, which can render the population estimate inaccurate. A direct comparison of trap capture of male western hemlock looper moths in sticky and non-saturating trap types showed that non-saturating traps captured significantly more males than similarly baited sticky traps at two different times in the flight period (Evenden et al. Reference Evenden, Borden, Van Sickle and Gries1995b). Sticky traps fashioned from milk cartons with a 695 cm2 sticky trapping surface became saturated when 40 Douglas-fir tussock moths were captured. Because the cumulative capture of 25 Douglas-fir tussock moths indicates incipient outbreaks, sticky traps can still be used to monitor population assessment of this species (Shepherd et al. Reference Shepherd, Daterman, Chorney and Gray1985). Non-saturating traps can retain large numbers of insects, which is particularly useful when traps remain in the field for the duration of the flight period. Even non-saturating Unitraps were less efficient in capturing western spruce budworm moths, Choristoneura occidentalis (Walsingham) (Lepidoptera: Tortricidae), when traps were not regularly maintained throughout the flight season (Sweeney et al. Reference Sweeney, McLean and Shepherd1990). Captured moths will decay if traps are deployed for long periods, and this can affect moth orientation to the trap (Sanders Reference Sanders1986b) and make the sample difficult to count. Non-saturating traps are more expensive than sticky traps but can be reused in subsequent seasons to reduce monitoring costs. However, pheromone contamination of the plastic non-saturating traps can persist across seasons (Grant Reference Grant1991) and influence subsequent trap capture.
Monitoring for population assessment implies that the number of insects captured in pheromone-baited traps reflects the actual population density of the target insect. This relationship is often established by relating the number of moths captured in pheromone-baited traps to estimates of population density of other life stages, or to defoliation caused by larval feeding. If this relationship is robust, pheromone-based monitoring can replace more costly sampling techniques such as foliage samples, or samples of immature stages (Sanders Reference Sanders1988). These types of relationships have been established for forest defoliators in Canada by sampling populations over long periods of time (Sanders Reference Sanders1988) or targeting populations of differing densities over a condensed period (Shepherd et al. Reference Shepherd, Daterman, Chorney and Gray1985; Sweeney et al. Reference Sweeney, McLean and Shepherd1990; Evenden et al. Reference Evenden, Borden and Van Sickle1995a).
There has been variable success in generating these relationships using sites at different population densities over short sample durations (two years). Trap capture of western hemlock looper moths in 10 µg-baited non-saturating traps was predictive of the number of eggs extracted from 100 g of arboreal lichen in the subsequent generation. This relationship was best at the beginning of the flight season (Evenden et al. Reference Evenden, Borden and Van Sickle1995a). Western spruce budworm season-long capture was not correlated to the number of larvae sampled from mid-crown branch tip samples of 50 trees at the same sites. However, capture of western spruce budworm moths per basal area or foliage biomass per ha significantly predicted larval density in the subsequent generation (Sweeney et al. Reference Sweeney, McLean and Shepherd1990). These results indicate that pheromone-baited traps are likely drawing in male moths from a distance far beyond where larval sampling was conducted. Trap capture of Douglas-fir tussock moths in saturating sticky traps baited with various pheromone doses did not predict numbers of egg masses or defoliation damage in the following generation, but trap capture of more than 25 moths was indicative of incipient outbreaks (Shepherd et al. Reference Shepherd, Daterman, Chorney and Gray1985).
There is a curvilinear relationship between the number of male forest tent caterpillar moths, Malacosoma disstria Hübner (Lepidoptera: Lasiocampidae), captured in non-saturating Unitraps and immature stages sampled within the same generation. Fewer male moths were captured in traps positioned in high-density sites than sites with intermediate densities (Jones et al. Reference Jones, Roland and Evenden2009). At extremely high population densities, it is possible that male moth orientation to pheromone is disrupted due to ambient levels of pheromone produced by the thousands of females in the stand or that males use cues other than olfaction to locate mates (Hagaman and Cardé Reference Hagaman and Cardé1984). It is also possible that characteristics of the defoliated stand alter the release dynamics of pheromone from the trap, which influence male moth response to the lure. Capture of male spruce budworm moths in pheromone-baited traps was variably related to larval density when sampled in many plots with different densities. However, moth capture was highly related to larval density when comparisons were made over time in a 21-year trapping study in the same location (Sanders Reference Sanders1988). This research resulted in the development of a season-long threshold capture of 50–100 moths to trigger more intensive larval sampling in an operational monitoring system (Sanders Reference Sanders1988).
The use of sex pheromone-baited traps for accurate measurement of population levels of forest defoliators has met with variable success. There is still work to do in developing the relationships between trap counts of adults and subsequent larval stages and ultimately, damage. These efforts may be assisted by incorporating additional information such as environmental conditions, parasitism levels and moth population quality into models relating moth trap capture to densities of immature stages and defoliation estimates. Information on moth population quality can be obtained from specimens captured in pheromone-baited traps. Measurement of moth size and assessment of disease status by moth dissection (Jones and Evenden Reference Jones and Evenden2008) can provide an indication of the stage of the outbreak cycle.
Monitoring for population quality: Pheromone trapping can also be used for ecological applications to better understand the population dynamics of forest defoliators. The effect of timing of adult emergence, geographic region, and population density on moth quality of the forest tent caterpillar and large aspen tortrix, Choristoneura conflictana Walker (Lepidoptera: Tortricidae) were studied (Jones and Evenden Reference Jones and Evenden2008) using moths captured in a combined pheromone-based monitoring system that targeted both species simultaneously (Jones et al. Reference Jones, Roland and Evenden2009). Moth quality was measured using wing area and microsporidian infection level of captured individuals. Wing area decreases over the flight season in forest tent caterpillar but not in large aspen tortrix moths captured in pheromone-baited traps (Jones and Evenden Reference Jones and Evenden2008). Comparisons of moths captured in different regions reveals that microsporidian infection of male forest tent caterpillar moths varies greatly with geographic region but not with population density. It is unclear whether defoliators with sublethal infections are as responsive to pheromone cues and therefore as likely to be captured in pheromone-baited traps as healthy moths. Dispersal of female but not male spruce budworm moths in the field was affected by infection with microsporidia (Eveleigh et al. Reference Eveleigh, Lucarotti, McCarthy, Morin, Royama and Thomas2007). Similarly, infected and uninfected male spruce budworm moths flew for similar durations in wind tunnel bioassays (Sanders and Wilson Reference Sanders and Wilson1990). However, the proportion of male western spruce budworm that exhibit pheromone-mediated behaviours in the wind tunnel was negatively correlated with the microsporidian load of male moths (Sweeney and McLean Reference Sweeney and McLean1987). This reduction in pheromone-mediated behaviours was not the result of reduced sensitivity to pheromone, as electroantennogram readings from the antennae of infected and uninfected males were similar (Sweeney and McLean Reference Sweeney and McLean1987). These types of data could be incorporated into models of population assessment and provide managers with a better understanding of the stage of the sampled population in the cyclical population dynamics of forest defoliators.
The mating status of male moths captured in pheromone-baited traps can also be determined in some species (Bergh and Seabrook Reference Bergh and Seabrook1986a). Dissection of male spruce budworm moths that were freshly caught in pheromone-baited sticky traps can be reliably used to determine recent mating activity by the colour of the secretion in section 7 of the ejaculatory duct primary simplex (Bergh and Seabrook Reference Bergh and Seabrook1986b). Using this tool, the proportion of mated males in the population can be tracked throughout the flight season or in different regions of the forest canopy (Bergh et al. Reference Bergh, Seabrook and Eveleigh1988). This tool could be adopted for studies of Allee effects on the population dynamics of the spruce budworm.
Mating disruption to reduce populations of forest defoliators
Some of the physical characteristics of forests and the biological attributes of forest defoliators predispose this system to effective management by mating disruption as part of an IPM approach. Pheromone-based mating disruption works best when pheromone is applied over a large area in an attempt to provide “area-wide management” (Witzgall et al. Reference Witzgall, Cork and Kirsch2010) and reduce damage at the edge of the treated area thought to result from immigration of gravid females into the treatment zone (Gut et al. Reference Gut, Stelinski, Thomson and Miller2004). Treatment of large areas of forested lands with aerially applied pheromone should be a good approach to achieve area-wide management, but penetration of the tree canopy to provide an even distribution of pheromone is problematic. Evenness of the forest canopy will promote better control by mating disruption because foliage will trap the pheromone within the stand and minimise the dissipation effect of the wind. The waxiness of conifer foliage may also promote the adherence of lipophilic pheromone molecules to the surface of the leaves where they can be adsorbed and re-released (Gut et al. Reference Gut, Stelinski, Thomson and Miller2004). The usefulness of mating disruption for control of forest defoliators may be limited by the fact that efficacy of pheromone-based control generally declines with increased population density (Gut et al. Reference Gut, Stelinski, Thomson and Miller2004).
Biological attributes that predispose some forest defoliators to control by mating disruption include: (1) a univoltine life cycle that results in a short and well-defined period of adult activity that can be targeted by pheromone application; (2) a restricted host range; and (3) limited dispersal capacity of females.
Spruce budworm: Thirty years of field and laboratory research conducted primarily in Canada on the feasibility of control of the spruce budworm by pheromone-based mating disruption led to the recent Canadian registration of Disrupt SBW microflakes® (Hercon Environmental, Emigsville, Pennsylvania, United States of America), a pheromone formulation that is applied aerially to affected forest stands. Laboratory and field studies on the development of pheromone-based mating disruption of spruce budworm conducted between 1974 and 2008 have recently been reviewed (Rhainds et al. Reference Rhainds, Kettela and Silk2012). Although most of the field studies reviewed demonstrated a reduction of mate-finding behaviour in pheromone-treated plots, there was rarely a difference in the number of egg masses sampled between control and treated areas. This potentially could be the result of the movement of gravid females into the treated area (Rhainds et al. Reference Rhainds, Kettela and Silk2012). Several studies illustrate that female spruce budworm moths are able to detect their own pheromone signal and increase their activity (Palaniswamy and Seabrook Reference Palaniswamy and Seabrook1978) and flight behaviours (Sanders Reference Sanders1987) in the presence of sex pheromone. Detection of high quantities of female-produced pheromones in heavily infested stands may be an adaptive cue that promotes female dispersal away from defoliated stands (Sanders Reference Sanders1987). The high dispersal capacity of female spruce budworm moths (Greenbank et al. Reference Greenbank, Schaefer and Rainey1980) has been cited as a constraint that may limit the effectiveness of mating disruption to control spruce budworm (Rhainds et al. Reference Rhainds, Kettela and Silk2012).
Limited success of early studies to develop the mating disruption technique to control spruce budworm led to a switch in research focus to understand the mechanisms by which pheromone interferes with mate-finding behaviour (Sanders Reference Sanders1982). There are several mechanisms to explain how pheromone-based mating disruption interferes with mate-finding behaviour in moth pests (Bartell Reference Bartell1982; Cardé Reference Cardé1990). These include: (1) false-trail-following, when male moths orient to synthetic sources of sex pheromone in a treated area instead of to females; (2) neurophysiological effects that result in desensitisation to the pheromone signal as a result of adaptation of antennal receptors or habituation of the central nervous system processing of the pheromone signal; and (3) camouflage of the female-produced pheromone plumes due to a physical masking by the synthetic pheromone in the environment. Careful experimentation of male spruce budworm moth behaviour in pheromone-mediated wind tunnels led to the conclusion that several mechanisms probably reduce mate-finding behaviour in pheromone-treated areas (Sanders Reference Sanders1995). Exposure to a concentration of 20 ng/m3 of pheromone caused habituation of male moths and reduced their ability to orient to females (Sanders Reference Sanders1996). False-trail-following is an important mechanism in mating disruption of spruce budworm moths, as disruption is greatest when the most attractive ratio of the two pheromone components is deployed (Sanders Reference Sanders1981). False-trail-following to synthetic pheromone plumes occurs repeatedly in wind tunnels treated with discrete pheromone plumes but did not completely prevent orientation to female-produced plumes (Sanders Reference Sanders1995).
Mating disruption of spruce budworm will be most beneficial as part of an IPM strategy with effective pheromone-based monitoring to warn forest managers of impending outbreaks (Sanders Reference Sanders1988) and additional control tactics such as aerial application of Bacillus thuringiensis subspecies kurstaki (Bacteria) at moderate to high budworm densities (Bauce et al. Reference Bauce, Carisey, Dupont and van Frankenhuyzen2004). Mating disruption will be most effective when incorporated for early intervention instead of a response to ongoing levels of measurable defoliation (Rhainds et al. Reference Rhainds, Kettela and Silk2012). This approach is similar to the highly successful “slow-the-spread” mating disruption programme for gypsy moth in the United States of America (Reardon et al. Reference Reardon, Leonard, Mastro, Leonhardt, McLane and Talley1998). Mating disruption is expected to work best at low population densities when non-pheromone-mediated chance encounters between males and females should be low (Gut et al. Reference Gut, Stelinski, Thomson and Miller2004). There is evidence that the efficacy of mating disruption declines with increased population density of spruce budworm moths, as mating of caged moth pairs increases with population density in a pheromone-treated environment but not in clean air (Palaniswamy et al. Reference Palanisawmy, Wiesner, Tan, Silk, Ross and Seabrook1982). This suggests that not only does mating disruption break down at high population densities, it may actually promote further mating at high densities. False-trail-following is the mating disruption mechanism that is most affected by high population densities and the mechanism most commonly cited to interfere with mating behaviour of the spruce budworm (Rhainds et al. Reference Rhainds, Kettela and Silk2012).
Male sensitivity to pheromone is a physiological characteristic that varies among moth species and has been linked to the susceptibility of moths to control by pheromone-based mating disruption (Gut et al. Reference Gut, Stelinski, Thomson and Miller2004). Male moths that exhibit a broad dose response to pheromone with no upper threshold are often more difficult to disrupt than species with a narrowly defined dose response that are arrested by high pheromone doses (Gut et al. Reference Gut, Stelinski, Thomson and Miller2004). Spruce budworm males orient to a wide range of pheromone doses in a wind tunnel and show no decrease in response to the highest dose tested (Sanders Reference Sanders1990). Males of the congener Choristoneura rosaceana Harris (Lepidoptera: Tortricidae), an important tree fruit pest, are also attracted to a wide range of pheromone doses (Gut et al. Reference Gut, Stelinski, Thomson and Miller2004). This species has also proven to be difficult to control by pheromone-based mating disruption (Lawson et al. Reference Lawson, Reissig, Agnello, Nyrop and Roelofs1996). Choristoneura rosaceana exhibits long-lasting adaptation of antennal sensory neurons in pheromone-treated atmospheres (Stelinski et al. Reference Stelinski, Gut and Miller2003). This may shield the central nervous system and prevent longer-lasting habituation of pheromone processing, which allows for rapid recovery of pheromone responsiveness in clean air (Evenden et al. Reference Evenden, Judd and Borden2000).
The chemical characteristics of the sex pheromone of each species can also affect the efficacy of pheromone-based mating disruption (Gut et al. Reference Gut, Stelinski, Thomson and Miller2004). The sex pheromone of the spruce budworm, like many other tortricine moths, consists of a blend of 14-carbon straight chain hydrocarbons with aldehyde, alcohol, or acetate functional groups and an internal double bond in the carbon-11 position (Silk et al. Reference Silk, Ross, Lonergan, Tan and Wiesner1980). The blend used in commercial formulations consists of a 95:5 ratio of (E):(Z)-11-tetradecenal. Each of these pheromone components has a molecular weight of 210.4, which corresponds to a rapid evaporation rate compared with pheromone products with longer chain lengths. Pheromone formulations with low molecular weight pheromone components will have a limited period of efficacy in the field. Wiesner et al. (Reference Wiesner, Fullarton, Tan and Silk1980) measured atmospheric concentration of pheromone in plots treated with hollow fiber dispensers releasing spruce budworm sex pheromone. Initial measurements of 9 ng/m3 decreased to < 2 ng/m3 over a two-day period (Wiesner et al. Reference Wiesner, Fullarton, Tan and Silk1980). It is important to note that even the initial release rate measured would not be adequate to habituate male spruce budworm moths (Sanders Reference Sanders1996).
Pheromone released from mating disruption formulations is in the vapour state but can condense and be adsorbed onto solid surfaces such as tree foliage under ordinary environmental conditions (Gut et al. Reference Gut, Stelinski, Thomson and Miller2004). The molecular weight and functional groups of the pheromone molecule will dictate the likelihood that pheromone adherence to foliage will occur. The “stickiness” of the pheromone molecule increases with carbon chain length and oxygenation of the hydrocarbons. Although partitioning of the pheromone from the vapour phase onto a solid surface can occur with pheromone molecules with > 200 MW (Gut et al. Reference Gut, Stelinski, Thomson and Miller2004), this effect would be expected to be greater for gypsy moth (19 carbons) and Douglas-fir tussock moth pheromones (21 carbons). Treatment with pheromones that are likely to adhere to the foliage can result in a buildup of pheromone concentration in the canopy and contribute to successful mating disruption (Gut et al. Reference Gut, Stelinski, Thomson and Miller2004).
Limitations to pheromone-based control of spruce budworm with mating disruption include: (1) high dispersal capacity of gravid female moths that may be influenced by perception of pheromone; (2) increased mating rates at high density in pheromone-treated air; (3) a broad dose response to pheromone by male moths; and (4) the physical properties of the relatively low molecular weight pheromone components.
Douglas-fir tussock moth: The use of pheromone-based mating disruption has also been studied for the Douglas-fir tussock moth, a defoliator of Douglas-fir, Pseudotsuga menziesii (Mirbel) Franco, true firs, Abies Miller, and pines, Pinus Linnaeus (Pinaceae) in western United States of America and British Columbia (Furniss and Carolin Reference Furniss and Carolin1977). The Douglas-fir tussock moth is a good species to target with pheromone-based mating disruption as the females are wingless and cannot disperse (Furniss and Carolin Reference Furniss and Carolin1977). The sex pheromone produced by female moths consists of a blend of 21-carbon ketones (Gries et al. Reference Gries, Grant, Gray, Hulme, Tracey and Gries1997), but only the main component, (Z)-6-heneicosen-11-one, is used in commercial applications. The long chain length of this compound prevents problems associated with premature loss of pheromone from dispensers (Sower et al. Reference Sower, Daterman, Funkhouser and Sartwell1983). Pheromone-mediated mating disruption interferes with moth reproduction at both low (Sower et al. Reference Sower, Daterman, Orchard and Sartwell1979) and high (Sower et al. Reference Sower, Daterman, Funkhouser and Sartwell1983) population densities. Although pheromone treatment reduced the number of egg masses produced in treated areas, it did not affect the rate of parasitism by three common egg parasitoids of the Douglas-fir tussock moth (Cook et al. Reference Cook, Wenz, Ragenovich, Reardon and Randall2005). Pheromone-mediated mating disruption of Douglas-fir tussock moth is compatible with naturally occurring mortality factors.
A series of experiments conducted in the early 1990s in southern British Columbia assessed a PVC bead pheromone formulation as a mating disruptant of the Douglas-fir tussock moth. Application of the high dose of 72 g/ha of pheromone applied from the air completely interfered with male moth orientation to feral females, and no egg masses were found in treated plots. Ground application also significantly reduced mating and egg production but did not eliminate it (Hulme and Gray Reference Hulme and Gray1994). The PVC beads continued to emit pheromone that impacted male moth behaviour up to two years after treatment, highlighting the long-lasting effect of the 21-carbon pheromone, (Z)-6-heneicosen-11-one (Gray and Hulme Reference Gray and Hulme1995). Later studies showed that the application rate of pheromone could be reduced by at least four-fold to 18 g/ha and still maintain 100% mating disruption (Hulme and Gray Reference Hulme and Gray1996).
Pheromones are mostly used for monitoring in management programmes for the Douglas-fir tussock moth, and a nuclear polyhedrosis virus is used for control (Shepherd Reference Shepherd1994). Effective mating disruption at low pheromone application rates (Hulme and Gray Reference Hulme and Gray1996) and long-lasting treatment effects (Gray and Hulme Reference Gray and Hulme1995) suggest that pheromone-mediated mating disruption may be an economical component in an IPM strategy for the Douglas-fir tussock moth. The economics of commercialisation of a mating disruption formulation for the Douglas-fir tussock moth may be improved by also using the same compound to disrupt mating of other tussock moth species (Grant Reference Grant1978; Grant and Frech Reference Grant and Frech1980).
Use of semiochemicals in management of bark and ambrosia beetles
The use of semiochemicals by bark and ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) is fundamentally different to the way in which they are used in the Lepidoptera (Borden Reference Borden1993). Bark and ambrosia beetles use a suite of semiochemicals to mediate orientation to hosts, aggregation and mass attack of hosts, and termination of mass attack (Borden et al. Reference Borden, Ryker, Chong, Pierce, Johnston and Oehlschlager1987a). Other members of the subcortical community, such as competitors and entomophagous insects, can also generate and respond to their own intraspecific signals or exploit interspecific semiochemicals in the environment (Borden Reference Borden1989). The volatile profile released by living trees changes after attack by bark beetles (Lusebrink et al. Reference Lusebrink, Evenden, Blanchet, Cooke and Erbilgin2011).
Exploitation of semiochemicals to manipulate bark and ambrosia beetle behaviour has been the focus of applied research and the goal of semiochemical-based management of these beetle pests in Canada (Borden Reference Borden1989). Most research has focussed on aggressive tree-killing bark beetles (Borden Reference Borden1992) that are pests of living stands and on ambrosia beetles that attack harvested trees (Borden and Lindgren Reference Borden and Lindgren1989). Control strategies include manipulation of semiochemical-based mass attack, exploitation of repellent semiochemicals such as antiaggregation pheromones and non-host volatiles to disrupt bark beetle orientation away from host trees, and enhancement of natural control (Borden and Lindgren Reference Borden and Lindgren1989). For each of these management strategies, there are various tactics that have been developed (Fig. 3), some of which have been applied to manage different bark beetle species under various stand conditions to address a range of stakeholder priorities in Canada.
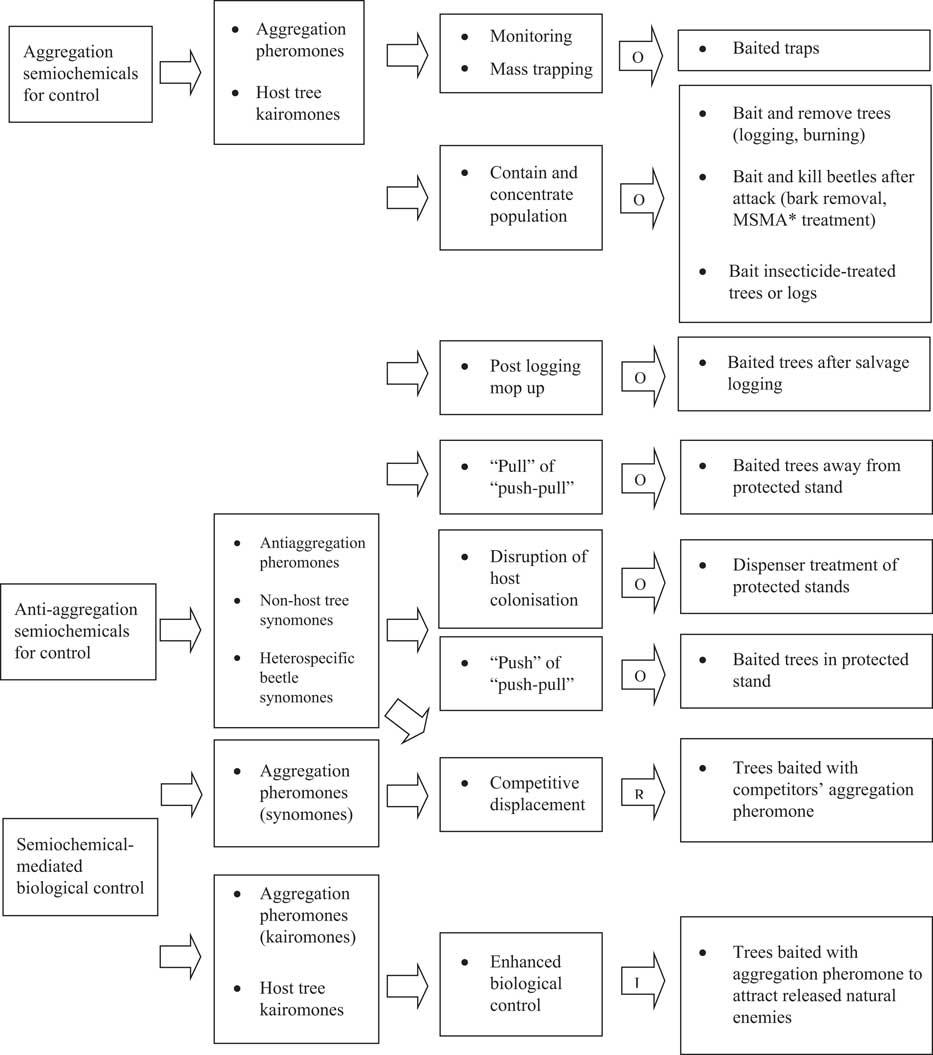
Fig. 3 Semiochemical-based management of bark and ambrosia beetles based on Borden (Reference Borden1989). Letters embedded in arrows indicate whether the tactics and tools are in the (O) operational, (R) research, or (I) innovation stage of the research-application continuum in Canada. *Monosodium methanearsonate (MSMA) was used operationally between the mid-1980s and 2004 but is no longer registered in Canada.
Host colonisation semiochemicals for monitoring and control of bark and ambrosia beetles
Monitoring: A major contribution of Canadian research to beetle management was the design of a multiple-funnel trap (Lindgren Reference Lindgren1983) that was later referred to as the Lindgren funnel trap. This trap features a series of funnels that provide a vertical silhouette to which dispensers of attractive semiochemicals can be fastened. Attracted beetles flying to the trap hit the angled sides of the funnels and are collected in a container fastened at the bottom of the series of funnels. This trap replaced more labour-intensive traps for bark and ambrosia beetles that used sticky surfaces for beetle capture (McLean and Borden Reference McLean and Borden1979). Processing time of collected beetles is reduced over earlier trap types, and the funnels collapse for easy storage and transport of the traps (Lindgren Reference Lindgren1983).
The Lindgren trap provides a dark, vertical silhouette with similar spectral reflectance properties to that of conifer tree hosts (Campbell and Borden Reference Campbell and Borden2005), which is an important component of host location for coniferophagous bark beetles (Campbell and Borden Reference Campbell and Borden2006). Attraction to aggregation pheromone-baited Lindgren funnel traps was reduced for three different species of bark beetles when the black funnels were replaced with white ones, suggesting that visual and olfactory cues are integrated in the host location process in bark beetles (Campbell and Borden Reference Campbell and Borden2006). Lindgren funnel traps have been widely adopted for research and management of many bark and ambrosia beetle species even though theoretical modelling exercises suggest that other trap types may be more efficient at beetle capture (Safranyik et al. Reference Safranyik, Shore and Linton2004). In empirical studies, the Lindgren funnel trap was more effective at capture of the striped ambrosia beetle Trypodendron lineatum (Olivier) (Coleoptera: Curculionidae: Scolytinae) than similarly baited drain pipe traps and slot traps in experiments conducted in British Columbia (McLean et al. Reference McLean, Bakke and Niemeyer1987).
Efficacious monitoring using semiochemical-baited traps also depends on development of an attractive semiochemical lure. In the case of bark beetles, development of semiochemical lures goes beyond identification of aggregation pheromones (Table 1) because, depending on the bark beetle species, kairomones released by host trees can be attractive alone or synergise beetle response to aggregation pheromone (Pureswaran and Borden Reference Pureswaran and Borden2005). Early investigations in British Columbia tested the attractiveness of the mountain pine beetle female-released aggregation pheromone, trans-verbenol, and the male-released aggregation pheromone, exo-brevicomin, in conjunction with six monoterpenes known to be released from the principal host of the mountain pine beetle, lodgepole pine, Pinus contorta Douglas (Pinaceae) (Conn et al. Reference Conn, Borden, Scott, Friskie, Pierce and Oehlschlager1983). These studies formed the basis for the development of commercial lures used to bait Lindgren funnel traps that released a combination of the two aggregation pheromones and the host kairomone myrcene (Borden et al. Reference Borden, Conn, Pierce, Oehlschlager and Chong1986). Lindgren funnel traps baited with this mixture of semiochemicals are used principally to monitor beetle emergence and the length of the flight period (Maclauchlan and Brooks Reference Maclauchlan and Brooks1998). This information can be used to restrict movement of infested logs during emergence and flight periods of the beetle (Borden and Lacey Reference Borden and Lacey1985).
Attraction of the mountain pine beetle is dependent on the dose of the pheromone (Miller et al. Reference Miller, Borden and Lindgren2005) and the kairomone myrcene (Borden et al. Reference Borden, Ryker, Chong, Pierce, Johnston and Oehlschlager1987a). The male aggregation pheromone, exo-brevicomin, is attractive at low doses and inhibitory at high doses (Borden et al. Reference Borden, Ryker, Chong, Pierce, Johnston and Oehlschlager1987a; Miller et al. Reference Miller, Borden and Lindgren2005). Interestingly, the shape of the dose response curve to trans-verbenol is dependent on the population density of beetles in the stand. At high population densities, the number of mountain pine beetles attracted to the female-released aggregation pheromone increased directly with pheromone release rate; at low population densities, response to the highest doses tested decreased (Miller et al. Reference Miller, Borden and Lindgren2005). Later research showed that the addition of another host volatile, terpinolene, to the mixture of aggregation pheromones and myrcene increased the attractiveness of the lure to mountain pine beetles and increased the female:male sex ratio of captured beetles, suggesting that baited traps might be developed for mass trapping (Borden et al. Reference Borden, Lafontaine and Pureswaran2008). With the expansion of the range of the mountain pine beetle into northern Alberta, semiochemical traps have been used to detect the presence of the beetle at the leading edge of the expansion (Table 2). Semiochemical-baited traps captured beetles released within a 500-m radius of the trap (Barclay et al. Reference Barclay, Safranyik and Linton1998), and therefore trap capture is a good indicator of presence of beetles in the direct trapping area.
Table 2 Semiochemical tactics used to manage the range expansion of mountain pine beetle into Alberta, Canada, 2007–2012 (Alberta Environment and Sustainable Resource Development, unpublished data).

Note: *Alberta Environment and Sustainable Resource Development.
Mass trapping: The best example of a successful, operational semiochemical-based mass-trapping programme in Canada is that designed to control the ambrosia beetles, Trypodendrum lineatum, Gnathotrichus sulcatus (LeConte), and Gnathotrichus retusus (LeConte) (Coleoptera: Curculionidae: Scolytinae) (Borden et al. Reference Borden, Chong, Gries and Pierce2001), which are pests of felled timber and freshly milled green lumber in British Columbia and the western United States of America (Borden Reference Borden1990). Beetles feed on ambrosia fungus and create tunnels stained by their fungal associates that lead to degradation of the lumber. Because of the different phenologies of the three main ambrosia beetle species, felled trees at sawmills and dryland sorting areas are vulnerable to attack for an eight-month period each year (Borden Reference Borden1990).
Research that led to the development of semiochemical-based mass trapping of ambrosia beetles was mainly conducted at Simon Fraser University and the University of British Columbia (British Columbia, Canada). Research began in the late 1960s with the identification of the aggregation pheromones of the three ambrosia beetle pests (Table 1). Further research showed that T. lineatum only responded to the (+) enantiomer (Borden et al. Reference Borden, Oehlschlager, Slessor, Chong and Pierce1980c) of the identified female-produced aggregation pheromone, lineatin (MacConnell et al. Reference MacConnell, Stokkink, Silverstein and Borden1977). As T. lineatum response to (+) enantiomer of lineatin is not influenced by the (−) enantiomer (Borden et al. Reference Borden, Oehlschlager, Slessor, Chong and Pierce1980c), a cheaper racemic mixture of the two enantiomers could be developed operationally for mass trapping.
The aggregation pheromone of G. sulcatus was identified as the male-produced sulcatol (Byrne et al. Reference Byrne, Borden, Stokkink, Swigar and Silverstein1974). Like lineatin, sulcatol is an optically active compound that has two enatiomers: S-(+)-sulcatol and R-(−)-sulcatol. The finding that the enatiomers of sulcatol act synergistically to attract G. sulcatus was published in Science in 1976 (Borden et al. Reference Borden, Chong, McLean, Slessor and Mori1976). Interestingly, G. retusus is also attracted to S-(+)-sulcatol (retusol) but species specificity of the chemical communication channel is maintained by arrestment of G. retusus to R-(−)-sulcatol (Borden et al. Reference Borden, Handley, McLean, Silverstein, Chong and Slessor1980b). Further, racemic mixtures of lineatin and sulcatol negatively influence response of T. lineatum and G. sulcatus (Shore and McLean Reference Shore and McLean1983). Beyond uncovering the interesting interspecific chemical communication among species of sympatric ambrosia beetles (Shore and McLean Reference Shore and McLean1983), this research led to the practical finding that purified S-(+)-sulcatol is required for semiochemical-based management of G. retusus, and separately baited traps are required for T. lineatum and G. sulcatus. In addition to aggregation pheromones, ambrosia beetles respond to host-produced kairomones, particularly ethanol (Moeck Reference Moeck1970). Ethanol and α-pinene are host-produced kairomones that attract ambrosia beetles to suitable hosts and also synergise response to aggregation pheromone (McLean and Borden Reference McLean and Borden1977; Borden et al. Reference Borden, Chong and Lindgren1980a). The unravelling of the chemical ecology of these three species of ambrosia beetles along with the invention of the Lindgren funnel trap (Lindgren Reference Lindgren1983), paved the way for operational studies of mass trapping for population control of ambrosia beetles in British Columbia.
Operational-level research was conducted between 1976 and 1981 that targeted control of G. sulcatus (McLean and Borden Reference McLean and Borden1979) and T. lineatum (Lindgren and Borden Reference Lindgren and Borden1983) by semiochemical-based mass trapping in conjunction with manipulation of stored log inventories. Traps are positioned to lure beetles away from the stored wood. As T. lineatum overwinters in the duff of the forest floor, traps are placed around the timber to be protected with the hope of intercepting beetles orienting to harvested logs. The Gnathotricus species overwinter in felled timber or woody debris and upon emergence need to be lured away from the stored wood. The success of mass trapping efforts was measured through overwintering samples of T. lineatum in the duff (Lindgren and Borden Reference Lindgren and Borden1983) and mark-recapture studies of both T. lineatum and G. sulcatus (Shore and McLean Reference Shore and McLean1988). As of 1990, there were more than 55 operational trapping programmes for ambrosia beetle in British Columbia and the western United States of America (Lindgren Reference Lindgren1990) with gross sales of ~$400 000 Canadian (E. Stokkink, Woodstock Management Inc., Nanaimo, British Columbia, Canada, personal communication) and an estimated benefit/cost ratio of 5:1 (Lindgren and Fraser Reference Lindgren and Fraser1994). Between 1995 and 2008, semiochemical-based management of ambrosia beetle on coastal Vancouver Island was steady between 20–35 sites with annual sales at ~$150 000–200 000 Canadian. In 2008, the economic downturn forced operators to reduce their stored log inventory, amalgamate sorting areas, and reduce spending on pest management. This led to a reduction in the number of operational mass trapping sites on Vancouver Island since 2008 to eight sites (E. Stokkink, Woodstock Management Inc., personal communication).
Containment and concentration: One of the most successful uses of semiochemicals in IPM against bark beetles is for the containment and concentration of populations, followed by tree removal or induction of beetle mortality (Fig. 3). This tactic exploits beetle aggregation pheromones and host tree kairomones to direct beetle attack to baited trees (Borden Reference Borden1992). This serves to intensify and direct the beetle attack into an area that can be easily reached after attack to remove the beetles. The approach also provides forest managers with much needed time in which to implement beetle removal from the stand before the emergence of the next generation of beetles, ~10–11 months later for univoltine species (Borden and Lacey Reference Borden and Lacey1985). In Canada, this tactic has been used against aggressive tree-killing bark beetles such as the mountain pine beetle, the Douglas-fir beetle, Dendroctonus pseudotsugae Hopkins, and the spruce beetle, Dendroctonus rufipennis Kirby (Coleoptera: Curculionidae: Scolytinae) (Borden Reference Borden1993). Canadian research on this semiochemical-mediated IPM tactic has mostly focussed on the mountain pine beetle, the spruce beetle, and the western balsam bark beetle, Dryocetes confuses Swaine (Coleoptera: Curculionidae: Scolytinae).
Early studies to assess the feasibility of the containment and concentration tactic against the mountain pine beetle focussed on the most appropriate mixture of semiochemicals to use in tree baits (Borden et al. Reference Borden, Conn, Friskie, Scott, Chong and Pierce1983c). As in the studies examining the attractiveness of various semiochemicals for the development of trap lures (Conn et al. Reference Conn, Borden, Scott, Friskie, Pierce and Oehlschlager1983), the most attractive tree baits released the two mountain pine beetle aggregation pheromones trans-verbenol and exo-brevicomin and the host kairomone myrcene (Borden et al. Reference Borden, Conn, Friskie, Scott, Chong and Pierce1983c). Although early research suggested that the incorporation of myrcene would improve tree baits releasing aggregation pheromones alone (Borden et al. Reference Borden, Conn, Friskie, Scott, Chong and Pierce1983c), later research showed that tree baits could be simplified to release only the aggregation pheromones without loss of efficacy (Borden et al. Reference Borden, Ebata, Maclauchlan, Hodgkinson, Chong and Lindgren1993). Currently available baits used for tree baiting contain only the two mountain pine beetle aggregation pheromones.
The tactic of semiochemical-based containment and concentration of mountain pine beetle infestations was designed following the protocol of Pitman (Reference Pitman1973) by baiting one lodgepole pine tree every 50 m in the treated areas (4 baits/ha) (Borden Reference Borden1992). These studies were assessed by comparing the ratio of the number of attacked trees during the year of tree baiting to that of previous years (green: red attack) in the treated and control plots (Borden et al. Reference Borden, Chong, Pratt and Gray1983b, Reference Borden, Conn, Friskie, Scott, Chong and Pierce1983c). Higher green: red attack ratios in the baited than control plots indicated containment of the beetle population either through restriction of dispersal out of the plot or attraction of beetles into the baited area (Borden Reference Borden1992). Concentration of beetle attack in response to semiochemical-based tree baiting was tested by dividing baited plots into concentric zones around a heavily infested plot centre (Gray and Borden Reference Gray and Borden1989). In the treated plots, the central zone of the plot contained nine baited trees at 50-m intervals. A middle zone consisted of a 50-m buffer around the perimeter of the central zone, and the exterior zone was a 100-m buffer around the middle zone. In baited plots, fresh beetle attack was concentrated within the central zone compared with control plots, in which the highest green: red ratio occurred in the middle zone due to beetle dispersal (Gray and Borden Reference Gray and Borden1989). The development of an attack-intensification ratio that incorporated the tree diameter and attack density in green: red attacked trees provided a metric that could be used in operational settings in the absence of unbaited control plots (Gray and Borden Reference Gray and Borden1989).
Operational use of tree baiting involves removal of the attacked trees before the next generation of beetles emerges (Fig. 3). This can be achieved by removal of the baited, attacked trees through sanitation-salvage logging after attack in the 11-month period before emergence of the next generation (Borden and Lacey Reference Borden and Lacey1985). Sanitation logging of infested trees is followed by debarking of harvested trees to kill the developing brood. The year following removal of the attacked pines, tree baits can be used to “mop-up” the residual mountain pine beetle population (Borden et al. Reference Borden, Chong and Fuchs1983a). If the infested area is too small to warrant salvage logging of the stand, tree baiting can still be used to concentrate beetle attack, followed by control of beetles in the baited trees by felling and burning, debarking, or insecticide application (Borden Reference Borden1990). Felling and burning is commonly used in British Columbia (Borden Reference Borden1990) and was adopted in Alberta, Canada (Table 2), where the invasion of large numbers of beetles into the province has resulted in adoption of semiochemical-based containment strategies (Table 2). When felling and burning of the attacked baited trees cannot be achieved due to the terrain or risk of wild fire, insecticide application to felled (Fuchs and Borden Reference Fuchs and Borden1985) or standing trees (Maclauchlan et al. Reference Maclauchlan, Borden, D’Auria and Wheeler1988) after attack can eliminate beetle populations in baited trees. The arsenical compound monosodium methanearsonate (MSMA) applied to the base of attacked trees through an axe frill (cut) in the bark is translocated up the tree. MSMA treatment kills beetles through direct poisoning, by indirectly decreasing phloem moisture and making the tree susceptible to fungal invasion (Maclauchlan et al. Reference Maclauchlan, Borden, D’Auria and Wheeler1988). Re-registration of MSMA was not pursued in 2005 due to findings of Canadian wild life research that showed treated trees that remain on the landscape change the foraging behaviour of insectivorous woodpeckers (Morrissey et al. Reference Morrissey, Dods and Elliott2008) and expose them to harmful levels of arsenic (Morrissey et al. Reference Morrissey, Albert, Dods, Cullen, Lai and Elliott2007).
Semiochemical-based containment and concentration have also been developed for other aggressive bark beetles as the result of Canadian research efforts. Tree baiting with the aggregation pheromone frontalin (Gries et al. Reference Gries, Pierce, Lindgren and Borden1988) and the host kairomone α-pinene has been used to contain and concentrate spruce beetle populations in living (Shore et al. Reference Shore, Hall and Maher1990) or dead (Gray et al. Reference Gray, Holsten and Pascuzzo1990) spruce “trap trees”. Tree baiting at 50-m intervals concentrated spruce beetle attack on standing spruce trees in semiochemical-baited plots compared with control plots under epidemic population densities (Shore et al. Reference Shore, Hall and Maher1990). Although baited trees did not receive more attacks than unbaited trees within treated plots, the overall concentration of attack was four times greater in treated versus control plots (Shore et al. Reference Shore, Hall and Maher1990). At endemic population densities, another approach to semiochemical-based management of spruce beetle is to bait living trees that are capable of defending themselves against the attracted beetles to avoid a population build-up in wind-thrown trees (Dyer and Safranyik Reference Dyer and Safranyik1977). One hundred randomly distributed spruce trees were baited in a 766-ha stand. Although all of the baited trees and some of the adjacent unbaited trees were attacked by spruce beetle, 95% of the attacks in the stand occurred on wind-thrown trees, suggesting that this approach is limited to stands with minimal downed host material (Dyer and Safranyik Reference Dyer and Safranyik1977). Later research on semiochemical-based management of the spruce beetle showed that the incorporation of other beetle-produced semiochemicals, including 4-methylene-6,6-dimethylbicyclo[3,1,1]hept-2-ene (verbenene) and 1-methyl-2-cyclohexen-1-ol (MCOL), into tree baits was variably effective depending on the geographic location of the experiment (Borden et al. Reference Borden, Gries, Chong, Werner, Holsten and Wieser1996).
Semiochemical-based containment and concentration of the western balsam bark beetle in subalpine fir, Abies lasiocarpa (Hooker) Nuttall (Pinaceae), stands can be achieved with tree baits releasing a racemic mixture of (±)-exo-brevicomin (Stock et al. Reference Stock, Pratt and Borden1994; Jeans-Williams and Borden Reference Jeans-Williams and Borden2006). Tree baiting experiments in 16-ha plots showed that western balsam bark beetle populations could be concentrated within 10 m of baited trees even at epidemic population densities (Stock et al. Reference Stock, Pratt and Borden1994). Concentration of beetle attack was better when two trees instead of one were baited per spot in one experiment (Stock et al. Reference Stock, Pratt and Borden1994) but not in another (Maclauchlan et al. Reference Maclauchlan, Brooks, Borden and Harder2003). Semiochemical-mediated response of the western balsam bark beetle is enantiospecific (Camacho et al. Reference Camacho, Pierce and Borden1993). Trees baited with a 9:1 blend of (+)-exo-brevicomin to (+)-endo-brevicomin were more heavily attacked than trees baited with (±)-exo-brevicomin (Camacho and Borden Reference Camacho and Borden1994), suggesting that enantiospecific tree baits may be more effective at concentrating beetle populations. However, later experiments that compared the ability of the standard bait and enantiospecific baits at different release rates to contain and concentrate western balsam bark beetle populations showed no difference in efficacy (Jeans-Williams and Borden Reference Jeans-Williams and Borden2006). Therefore, the cheaper racemic mixture of (±)-exo-brevicomin is an effective tree bait that can be used to manage the western balsam bark beetle for timber or wildlife values (Maclauchlan et al. Reference Maclauchlan, Brooks, Borden and Harder2003). Sympatric distribution of the western balsam bark beetle and the spruce beetle and lack of overlapping pheromone components allows for tree baiting targeting both species in stands of spruce and subalpine fir (Greenwood and Borden Reference Greenwood and Borden2000). In 9-ha plots treated with tree baits of both species, semiochemical treatment was successful at concentrating and containing beetle activity of both species. Green to red attack ratios on both species of trees were greatest in the baited central zone of plots compared with the unbaited peripheral zones, suggesting that populations of both bark beetle species can be simultaneously manipulated (Greenwood and Borden Reference Greenwood and Borden2000).
Containment and concentration of bark beetle populations in trees baited with semiochemicals followed by beetle removal (Fig. 3) has become a mainstay of forest pest management. However, a comparison of semiochemical techniques to remove Douglas-fir beetle from the population suggested that more beetles could be removed with semiochemical-baited traps than with baited living or trap trees (Laidlaw et al. Reference Laidlaw, Fabris, Wieser, Prenzel and Reid2003). Baited trees became saturated with beetles within the first 20–30 days of beetle flight activity, whereas traps continued to remove beetles from the population throughout the flight duration (Laidlaw et al. Reference Laidlaw, Fabris, Wieser, Prenzel and Reid2003). This finding suggests that mass trapping of bark beetles in limited and particularly environmentally sensitive areas may be a viable alternative to tree baiting that involves the use of chemicals or tree removal (Borden Reference Borden1993).
Antiaggregation semiochemicals for control of bark and ambrosia beetles
Bark and ambrosia beetles also use semiochemical messages to interrupt or avoid aggregation and host colonisation processes. These semiochemicals can include antiaggregation pheromones or heterospecific synomones that regulate the density of the mass attack by deterring late-arriving beetles from attacking a “full” tree of conspecifics or heterospecifics, respectively (Borden Reference Borden1997). In addition, most coniferophagous bark (Huber et al. Reference Huber, Gries, Borden and Pierce2000) and ambrosia (Borden et al. Reference Borden, Chong, Gries and Pierce2001) beetles are able to sense semiochemicals emitted from non-host angiosperm trees, which is adaptive as beetles can avoid orientation to non-hosts. There is understandably great interest in the exploitation of antiaggregation behaviour of bark beetles to disrupt mass attack and avoid host tree death (Borden Reference Borden1997). This has led to extensive applied research on the identification and use of these compounds in semiochemical-based management of bark and ambrosia beetles.
Antiaggregation pheromones: Most Canadian research on antiaggregation pheromones has focussed on verbenone (4,6,6-trimethylbicyclo [3,1,1]-hetp-3-en-2-one) as it elicits an antiaggregation response by several species of bark beetle and other phloeophagous insects (Lindgren and Miller Reference Lindgren and Miller2002). When tested in Lindgren funnel traps baited with aggregation pheromones, verbenone significantly reduced trap capture of the mountain pine beetle, and two secondary bark beetles that attack pine, the pine engraver, Ips pini Say and I. latidens (LeConte) (Coleoptera: Curculionidae: Scolytinae) (Miller et al. Reference Miller, Lindgren and Borden1995). In the mountain pine beetle, verbenone is produced by autooxidation (Hunt et al. Reference Hunt, Gries, Lindgren and Borden1989) and oxidation of verbenol by microbial associates of the female beetles (Hunt and Borden Reference Hunt and Borden1990) late in the colonisation process (Pureswaran et al. Reference Pureswaran, Gries, Borden and Pierce2001). Therefore, this compound may better be described as a kairomone that signals the breakdown of plant tissues (Lindgren and Miller Reference Lindgren and Miller2002) and hence elicits broad antiaggregation response from insects that rely on relatively fresh plant tissue for survival and brood production.
Early studies to explore the usefulness of verbenone in the pest management of the mountain pine beetle looked promising (Lindgren et al. Reference Lindgren, Borden, Cushon, Chong and Higgins1989). Experiments in which lodgepole pine trees were baited with verbenone (5–8 mg/day) with or without attractive semiochemical baits showed that the number of mass-attacked trees was reduced and the aggregation process was interrupted in the verbenone-treated plots (Lindgren et al. Reference Lindgren, Borden, Cushon, Chong and Higgins1989). These early experiments were conducted at low to moderate mountain pine beetle pressure, which may have promoted the success of the verbenone treatments (Bentz et al. Reference Bentz, Kegley, Gibson and Their2005; Progar Reference Progar2005). Tree baiting experiments using verbenone (10 mg/day) and only the male-produced aggregation pheromone exo-brevicomin showed that verbenone masked the attractive effect of the exo-brevicomin baits, which resulted in verbenone-treated trees escaping mass attack (Shore et al. Reference Shore, Lindgren and Safrankyik1992). Later field applications of verbenone against the mountain pine beetle were inconsistent (Borden Reference Borden1997 and references therein) even when verbenone was released at 20 mg/day (Safranyik et al. Reference Safranyik, Shore, Linton and Lindgren1992), perhaps in part due to the transformation of verbenone under ultraviolet radiation to the inactive by-product chrysanthenone (Kostyk et al. Reference Kostyk, Gries and Borden1993). More recent studies illustrated that high release rates of verbenone (>25 mg/day) improve its effectiveness in the protection of lodgepole pine from attack by the mountain pine beetle (Borden et al. Reference Borden, Huber, Earle and Chong2003), and this formulation was registered in Canada in 2006 (Borden et al. Reference Borden, Sparrow and Gervan2007). Trees are further protected if high doses of verbenone are combined with the volatiles released from the bark of non-host angiosperm trees (Borden et al. Reference Borden, Huber, Earle and Chong2003). Verbenone treatment at the high release rate (>25 mg/day) successfully protected high-value residential pine trees from mass attack by the mountain pine beetle as part of an IPM programme that included disposal of infested trees (Borden et al. Reference Borden, Sparrow and Gervan2007).
Non-host angiosperm tree volatiles: As the results of tree baiting experiments that tested verbenone as a bark beetle antiaggregant were mixed (Borden Reference Borden1997), research efforts shifted toward investigation of different semiochemicals that could potentially interfere with semiochemical-mediated aggregation of bark beetles. Wilson et al. (Reference Wilson, Gries, Gries and Borden1996) showed in electrophysiological studies that mountain pine beetle antennae are responsive to six carbon alcohols that are ubiquitous green leaf volatiles produced by the leaves of angiosperm trees. Traps baited with the antennally active green leaf volatiles in combination with an attractive mountain pine beetle lure significantly reduced trap capture of both male and female beetles compared with that in traps baited with the mountain pine beetle lure alone. Disruption of the aggregation process was highest when the green leaf volatiles were presented as a mixture (Wilson et al. Reference Wilson, Gries, Gries and Borden1996). The two most effective green leaf volatiles, (E)-2-hexen-1-ol and (Z)-3-hexen-1-ol, reduced trap capture in traps baited with the mountain pine beetle lure to a similar degree as verbenone (Wilson et al. Reference Wilson, Gries, Gries and Borden1996). Trees baited with these green leaf volatiles in combination with the attractive mountain pine beetle bait experienced a significantly lower density of attack than trees baited with the attractive bait alone and the level of attack was similar to that of nonbaited trees (Wilson et al. Reference Wilson, Gries, Gries and Borden1996). Green leaf volatiles also disrupt the aggregation response of the western pine beetle, Dendroctonus brevicomis (LeConte) (Coleoptera: Curculionidae: Scolytinae), and the spruce beetle at low (Poland et al. Reference Poland, Borden, Stock and Chong1998) but not high (Huber and Borden Reference Huber and Borden2003) population densities.
In addition to green leaf volatiles, bark volatiles of non-host angiosperm trees may provide important semiochemical information to host-seeking coniferophagous bark beetles. The volatile blends emitted from six angiosperm trees were collected and pulsed over the antennae of five different species of coniferophagous bark beetles in coupled gas chromatographic-electroantennographic detection analyses (Huber et al. Reference Huber, Gries, Borden and Pierce2000). Of the 25 antennally active compounds recovered, six elicited a response from the antennae of all five bark beetle species tested (Huber et al. Reference Huber, Gries, Borden and Pierce2000), suggesting that in-flight assessment of non-hosts might be an important aspect of host foraging in coniferophagous bark beetles. Incorporation of non- host bark volatiles into lures emitted from traps reduced the response of the western balsam bark beetle to traps baited with attractive semiochemicals of this species (Huber and Borden Reference Huber and Borden2003). A blend of bark volatiles enhanced the antiaggregation response of the green leaf volatiles to mountain pine beetle (Huber and Borden Reference Huber and Borden2003). A blend of four compounds identified from the bark of trembling aspen, Populus tremuloides (Michaux) (Salicaceae), disrupted mountain pine beetle response to attractant-baited traps by 98%. Of the four bark compounds from trembling aspen that elicit antennal response by the mountain pine beetle, only 1-hexanol was behaviourally active alone and reduced beetle capture in traps baited with attractive semiochemicals (Borden et al. Reference Borden, Wilson, Gries, Chong, Pierce and Gries1998). Conophthorin, a spiroketal that is present in the bark of several nonhost angiosperm trees (Huber et al. Reference Huber, Gries, Borden and Pierce1999), reduced trap capture of Douglas-fir beetle in attractant-baited Lindgren funnel traps (Huber and Borden Reference Huber and Borden2001a). In general, blends of non-host bark volatiles disrupted the aggregation behaviour of bark beetles to a greater extent than individual compounds (Borden et al. Reference Borden, Wilson, Gries, Chong, Pierce and Gries1998; Huber and Borden Reference Huber and Borden2001b, Reference Huber and Borden2003).
Non-host leaf and bark volatiles, with and without the antiaggregation pheromone verbenone, have been tested as protectants of lodgepole pine trees from attack by the mountain pine beetle (Huber and Borden Reference Huber and Borden2001b). A smaller proportion of trees baited with blends of non-host bark and green leaf volatiles or verbenone, in addition to an attractive mountain pine beetle bait, were mass attacked compared with trees baited with the attractive bait alone (Huber and Borden Reference Huber and Borden2001b). Disruption of the aggregation response of mountain pine beetle was greatest when trees were baited with a nine-component blend of non-host bark and leaf volatiles plus verbenone. This was the case when the plume of the disruptant semiochemicals overlapped or was spatially distinct from that of the attractant semiochemicals (Huber and Borden Reference Huber and Borden2001b). Protection of lodgepole pine from attack by mountain pine beetle was further improved by combining a slightly simpler seven-component blend of non-host bark and leaf volatiles with high doses of verbenone (Borden et al. Reference Borden, Huber, Earle and Chong2003). Grid baiting at 10-m intervals with the antiaggregation semiochemicals reduced the incidence and density of attack by mountain pine beetle compared with control plots without disruptants (Borden et al. Reference Borden, Huber, Earle and Chong2003). Disruption of aggregation in response to these repellent semiochemicals would likely work best as part of a “push-pull” management system (defined later) when beetle population densities are low (Borden et al. Reference Borden, Huber, Earle and Chong2003).
Nonhost bark volatiles have also been tested as repellents to ambrosia beetles (Borden et al. Reference Borden, Chong, Gries and Pierce2001). The antennal receptors of male and female T. lineatum repeatedly responded to six volatile compounds released from angiosperm (non-host) bark. These same compounds were sensed by antennae of two other ambrosia beetle species, G. sulcatus and G. retusus (Borden et al. Reference Borden, Chong, Gries and Pierce2001). Non-host volatiles positioned in multiple-funnel traps interfered with capture of T. lineatum in attractive lineatin-baited traps only when blends of two or more non-host compounds were used. Individual non-host volatiles did not interfere with T. lineatum orientation to lineatin-baited traps (Borden et al. Reference Borden, Chong, Gries and Pierce2001). Application of a blend of four non-host volatiles (1-hexanol, benzyl alcohol, methyl salicylate, salicylaldehyde) to unattacked logs significantly reduced the number of landings by all three species of ambrosia beetles early in the season. Use of non-host volatiles for log protection would need to be incorporated with another pest management tactic to achieve season-long control. Pairing repellent and attractive semiochemicals in a push-pull system or the use of other repellent visual or gustatory cues should be tested in this system to enhance operational semiochemical-based control of ambrosia beetles (Borden et al. Reference Borden, Chong, Gries and Pierce2001).
Heterospecific synomones: It is adaptive for insects to detect semiochemicals produced and released by heterospecifics to avoid costly attempts at orientation or aggregation in response to the wrong species or to a resource already occupied by a different species. This would be particularly important for sympatric species that have overlapping pheromone components (Evenden et al. Reference Evenden, Judd and Borden1999) as is the case for many bark beetles (Pureswaran et al. Reference Pureswaran, Gries and Borden2004). Measurement of antennal response of the mountain pine beetle, Douglas-fir beetle, spruce beetle, and and the western balsam bark beetle showed that eight beetle-produced compounds elicited a response from the antennae of all species (Pureswaran et al. Reference Pureswaran, Gries and Borden2004), suggesting that beetles are capable of detecting heterospecifics. Combining heterospecific compounds with attractive semiochemical lures can disrupt the aggregation behaviour of the target species. Capture of spruce beetles in Lindgren funnel traps baited with attractive semiochemicals was reduced when traps were additionally baited with the aggregation pheromones of two secondary bark beetles commonly associated with the spruce beetle, Ips tridens Mannerheim and Dryocetes affaber Mannerheim (Coleoptera: Curculionidae: Scolytinae) (Poland and Borden Reference Poland and Borden1998a). Similarly, trap capture of the pine engraver was reduced when traps baited with their aggregation pheromone, ipsdienol, simultaneously released the aggregation pheromone of the heterospecific Ips latidens, ipsenol (Borden et al. Reference Borden, Devlin and Miller1992). Trapping experiments targeting mountain pine beetle showed that aggregation behaviour could be disrupted by release of 3-methyl-2 cyclohexen-1-one (MCH), an antiaggregation pheromone of the Douglas-fir and spruce beetles, from traps baited with semiochemicals attractive to the mountain pine beetle (Borden et al. Reference Borden, Poirier and Pureswaran2004).
Attempts to use heterospecific synomones in pest management of aggressive bark beetles have focussed on two tactics: disruption of host colonisation and competitive displacement (Fig. 3). Disruption of host colonisation was tested in tree-baiting experiments targeting the mountain pine beetle in which the heterospecific synomone MCH was used to bait trees in addition to attractive semiochemicals. The MCH treatment significantly reduced the percentage of lodgepole pine trees that were mass attacked by the mountain pine beetle compared with trees that were baited with the attractive semiochemicals alone (Borden et al. Reference Borden, Poirier and Pureswaran2004). However, the level of disruption provided by MCH-baited trees was not as great as that provided by verbenone alone, and the combination of MCH with verbenone and another mountain pine beetle antiaggregation pheromone, 2-phenyl ethanol, did not enhance the activity of verbenone alone (Borden et al. Reference Borden, Poirier and Pureswaran2004).
Competitive displacement works on the premise that colonisation of the host tree by a secondary bark beetle species can reduce the fitness of the aggressive tree-killing species through competition for resources (Borden Reference Borden1992). Pheromone baits of the pine engraver, the secondary bark beetle species, induce attack on trees that have been mass attacked by the mountain pine beetle (Rankin and Borden Reference Rankin and Borden1991; Safranyik et al. Reference Safranyik, Shore and Linton1996). Co-colonisation by both species of bark beetle reduced the number of mountain pine beetle progeny by 72.5% compared to that of trees naturally attacked by mountain pine beetle alone (Rankin and Borden Reference Rankin and Borden1991). Similarly, offspring production was reduced when logs attacked by the spruce beetle were subsequently baited with pheromones to attract secondary bark beetles. This reduction was not great enough to encourage semiochemical-mediated competitive displacement as a potential pest management tool for spruce beetle (Poland and Borden Reference Poland and Borden1998b). Semiochemical-based competitive displacement has not been operationally adopted but may have potential especially to slow the spread of mountain pine beetle attack by inducing competition for phloem resources with the pine engraver (Rankin and Borden Reference Rankin and Borden1991).
Push-pull management strategy
Exploitation of bark beetle aggregation behaviour and antiaggregation behaviour can be combined to manage bark beetle populations with a “push-pull” tactic (Fig. 3). The push-pull tactic was first tested against the mountain pine beetle using aggregation pheromones plus the attractive host kairomone myrcene to pull beetles toward baited trees and the antiaggregation pheromone verbenone to push beetles away from protected trees (Lindgren and Borden Reference Lindgren and Borden1993). In an attempt to protect trees in a central zone of each plot, 75 dispensers releasing 5 mg/day of verbenone were positioned evenly throughout a 150×50 m plot using a 10×10 m grid pattern in treated plots. In central plot zones treated with verbenone, a lower percentage of trees were mass attacked compared with central zones that were untreated (Lindgren and Borden Reference Lindgren and Borden1993). Treatment of the zones surrounding the central zones with attractive semiochemicals resulted in a shift in attack distribution so that >2 times as many trees were attacked in the pull zones compared with the central push zone. This ratio was only maintained when semiochemicals mediating both antiaggregation and aggregation behaviour were deployed (Lindgren and Borden Reference Lindgren and Borden1993).
Later operational trials tested some modifications of the push component of the tactic: (1) the combination of non-host volatiles with high doses of verbenone in the absence of the pull component; and (2) the density of antiaggregants in the push component in combination with a surrounding pull component composed of trees baited with aggregation pheromone and myrcene (Borden et al. Reference Borden, Burleigh and Birmingham2006). Plots treated with high doses of verbenone or nonhost volatiles incurred less attack than areas surrounding the treatment zone but a combination of both types of antiaggregation semiochemicals did not enhance the protection (Borden et al. Reference Borden, Burleigh and Birmingham2006). The density of antiaggregants in the centrally located 1-ha push component of a 4-ha plot significantly influenced the green: red tree attack ratio, which was lowest in plots receiving the antiaggregants at densities of 44–100 dispensers per ha (Borden et al. Reference Borden, Burleigh and Birmingham2006). However, protection was not achieved in all replicates that received both the push and pull treatments, and this was attributed to high beetle pressure at the time the experiments were conducted (Borden et al. Reference Borden, Burleigh and Birmingham2006). These experiments culminated in a recommendation for operational use of the push-pull tactic with high-dose verbenone dispensers that release 100 mg/day positioned at 10–15 m centres in combination with attractive semiochemicals to concentrate attack and make subsequent removal of infested trees easier. These authors stress that semiochemical-based management of bark beetle populations must be part of an IPM programme that also removes infested trees from the landscape. They provide guidelines of how to use the push-pull tactic when: (1) the density of lodgepole pine is >400 per ha; (2) the mean diameter at breast height of host trees is ⩽25 cm; and (3) the current level of attack in the stand is ⩽15% (Borden et al. Reference Borden, Burleigh and Birmingham2006).
Use of semiochemicals in detection and management of invasive species
One of the most important uses of semiochemicals in IPM of forest insects is for the early detection of invasive species. Invasive insects are often present initially at low population densities in the new habitat and can be difficult to detect. Semiochemical-baited traps are particularly useful for monitoring insects at low population density (Witzgall et al. Reference Witzgall, Cork and Kirsch2010) because the olfactory system of insects is so highly tuned to specific chemical cues in the environment. Surveillance programmes for wood boring beetles at ports and in urban environments use Lindgren funnel traps baited with commercially available attractants that are attractive to several species of wood borers (Humble et al. Reference Humble, Allen, Hurley, Sun, Gao and Gill2003). This approach is useful because the pest manager does not need to know the species identity of the invasive wood borers before trap placement, as kairomone-baited traps attract several wood boring beetles. In many instances, detection of invasive insects dictates immediate management action (Nealis and Erb Reference Nealis and Erb1993), and in some cases, semiochemical-based management can be effective at slowing the spread of recently introduced pests (Reardon et al. Reference Reardon, Leonard, Mastro, Leonhardt, McLane and Talley1998). Canadian research on the chemical ecology of invasive species ranges from the identification of semiochemicals (Table 1) to the implementation of semiochemicals for monitoring for detection and spread of populations and for control of those species that become established in Canada.
The brown spruce longhorned beetle
The invasive brown spruce longhorned beetle, Tetropium fuscum (Fabricius) (Coleoptera: Cerambycidae), was first discovered in mature, apparently healthy, red spruce, Picea rubens Sargent (Pinaceae), in Point Pleasant Park, Halifax, Nova Scotia, Canada (Smith and Hurley Reference Smith and Hurley2000). In its native Europe, the brown spruce longhorned beetle breeds primarily in stressed or dying Norway spruce, Picea abies (Linnaeus) Karst. (Juutinen Reference Juutinen1955) and is sympatric with T. castaneum (Linnaeus), which also infests stressed or damaged spruce, as well as Pinus and Abies (Sama Reference Sama2002); Tetropium castaneum is not known to be established in North America. In Nova Scotia, the brown spruce longhorned beetle infests red spruce, white spruce (P. glauca (Moench) Voss), black spruce (P. mariana (Miller) Britton, Sterns, and Poggenburg), and Norway spruce (Smith and Humble Reference Smith and Humble2001; O’Leary et al. Reference O’Leary, Hurley, MacKay and Sweeney2003) and has been detected in 11 counties (Cunningham Reference Cunningham2010). In its new range, the brown spruce longhorned beetle overlaps with the transcontinental, Nearctic T. cinnamopterum Kirby, which infests dying or recently felled spruce and occasionally pines (Furniss and Carolin Reference Furniss and Carolin1977). The brown spruce longhorned beetle is now an important quarantine pest of spruce in Canada.
The chemical ecology of the brown spruce longhorned beetle has been recently described. Stress-induced compounds in spruce, such as the sesquiterpenes and monoterpenes, are important host kairomones used for host location by beetles (Sweeney et al. Reference Sweeney, de Groot, MacDonald, Smith, Cocquempot and Kenis2004, Reference Sweeney, de Groot, Price and Gutowski2006). Attractants for the brown spruce longhorned beetle and the native T. cinnamopterum have been identified as a result of Canadian research (Silk et al. Reference Silk, Sweeney, Wu, Price, Gutowski and Kettela2007; Sweeney et al. Reference Sweeney, Silk, Gutowski, Wu, Lemay and Mayo2010). A blend of spruce monoterpenes (Sweeney et al. Reference Sweeney, de Groot, MacDonald, Smith, Cocquempot and Kenis2004) and the male-produced aggregation pheromone (“fuscumol”; (S)-(E)-6, 10-dimethyl-5, 9-undecadien-2-ol), can be used to trap the brown spruce longhorned beetle and T. cinnamopterum in Canada and T. castaneum in Europe (Silk et al. Reference Silk, Sweeney, Wu, Price, Gutowski and Kettela2007; Sweeney et al. Reference Sweeney, Silk, Gutowski, Wu, Lemay and Mayo2010). This technology is now in operational use by Canadian Provinces, the United States of America, and the Canadian Food Inspection Agency as a detection tool to measure the annual spread of the brown spruce longhorned beetle. There is some evidence that the spatial spread of this insect is limited (Rhainds et al. Reference Rhainds, Mackinnon, Porter, Sweeney and Silk2011), which bodes well for incorporating attractive semiochemicals into a control strategy (Fig. 1). Pheromone-based mating disruption using aerially applied Hercon flakes containing synthetic fuscumol has been tested for suppression of brown spruce longhorned beetle populations to slow its spread. Mating disruption trials by the Canadian Forest Service have proven effective, and registration of this technology is imminent (P.J.S., personal observation); no other technology to detect or control/slow the spread of this insect is presently available, so this development is of critical importance.
Canadian research on the chemical ecology of the brown spruce longhorned beetle has also led to fundamental basic discoveries on its reproductive biology and physiology. The “calling” posture of male beetles correlates with the release of pheromone. The presence of other males (chorussing) stimulates calling in the brown spruce longhorned beetle, which suggests that individuals evaluate intrasexual competition and modify pheromone output under competition (Lemay et al. Reference Lemay, Silk and Sweeney2010). Stable isotope labelling studies (Mayo et al. Reference Mayo, Silk, Cusson and Béliveau2013) showed that the pheromone is biosynthesised de novo via the mevalonate pathway, mainly in the midgut of male beetles. To further verify this hypothesis, a key mevalonate pathway enzyme, farnesyl diphosphate synthase, was cloned from the brown spruce longhorned beetle. Farnesyl diphosphate synthase generates the sesquiterpene farnesyl diphosphate, which is the immediate precursor of farnesol and is therefore required for the synthesis of fuscumol. Experiments assessing the impact of farnesyl diphosphate synthase-directed RNA interference on fuscumol production should help clarify the role of the mevalonate pathway in fuscumol biosynthesis.
The brown spruce longhorned beetle and T. cinnamopterum also use contact pheromones identified as cuticular hydrocarbons 11-methylheptacosane (S11- more active than R11-methylheptacosane) and (9Z)-pentacosene, respectively (Silk et al. Reference Silk, Sweeney, Wu, Sopow, Mayo and Magee2011b). Both pheromones release conspecific precopulatory and copulatory activity after antennal contact.
The emerald ash borer
The emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), has killed millions of ash (Fraxinus Linnaeus) (Oleaceae) trees in southwestern Ontario, Canada, Michigan, United States of America, and surrounding states (Cappaert et al. Reference Cappaert, McCullough, Poland and Siegert2005). It poses a major economic and environmental threat to urban and forested areas in Canada and the United States of America. Early detection of emerald ash borer infestations has proven very difficult. Visual signs and symptoms, such as D-shaped exit holes on the main stem, epicormic shoots, bark deformities, and thinning crowns, usually appear only on heavily infested trees a year or more after populations have been established (de Groot et al. Reference de Groot, Grant, Poland, Scharbach, Buchan and Nott2008). Combined research efforts of Canadian and American researchers have led to progress in the development of traps and lures for detection of this pest. Both visual cues, such as the colour purple (Francese et al. Reference Francese, Mastro, Oliver, Lance, Youssef and Lavalee2005, Reference Francese, Fraser, Lance and Mastro2007) and cues produced by conspecifics (Lelito et al. Reference Lelito, Boeroeczky, Jones, Fraser, Mastro and Tumlinson2009), and semiochemical cues have been identified. Emerald ash borer adults are attracted to girdled ash trees (Poland et al. Reference Poland, de Groot, Grant, Macdonald and McCullough2004, Reference Poland, McCullough, de Groot, Grant, MacDonald and Cappaert2005), green leaf volatiles from ash foliage (Rodriguez-Saona et al. Reference Rodriguez-Saona, Poland, Miller, Stelinski, Grant and de Groot2006; de Groot et al. Reference de Groot, Grant, Poland, Scharbach, Buchan and Nott2008), and bark sesquiterpenes emitted by stressed ash (Crook et al. Reference Crook, Krimian, Francese, Fraser, Poland and Sawyer2008). Crook et al. (Reference Crook, Krimian, Francese, Fraser, Poland and Sawyer2008) identified five antennally active compounds from the bark of green ash, Fraxinus pennsylvanica Marshall (Oleaceae): α-cubebene, α-copaene, 7-epi-sesquithujene, trans-β-caryophyllene, and α-humulene. Many of these components occur in commercially available Manuka oil, except 7-epi-sesquithujene, which, together with the aforementioned compounds, can be found in Phoebe oil (Crook et al. Reference Crook, Krimian, Francese, Fraser, Poland and Sawyer2008). Purple prism traps baited with Phoebe oil captured more emerald ash borers than unbaited purple traps (Crook et al. Reference Crook, Krimian, Francese, Fraser, Poland and Sawyer2008). However, with the exception of the rather labour-intensive girdled ash tree method (Poland and McCullough Reference Poland and McCullough2006), most of these traps and lures have been tested in areas of high emerald ash borer populations, so their efficacy at detecting low populations in the first year of infestation is unknown.
The chemical ecology of the emerald ash borer has been studied since its detection in North America (Crook and Mastro Reference Crook and Mastro2010). Two types of host volatiles are attractive to adults: green leaf volatiles (Poland et al. Reference Poland, de Groot, Grant, Macdonald and McCullough2004, Reference Poland, McCullough, de Groot, Grant, MacDonald and Cappaert2005, Reference Poland, Rodriguez-Saona, Grant, Buchan, de Groot and Miller2006, Reference Poland, Pureswaren, Grant and de Groot2007; Rodriguez-Saona et al. Reference Rodriguez-Saona, Poland, Miller, Stelinski, Grant and de Groot2006; de Groot et al. Reference de Groot, Grant, Poland, Scharbach, Buchan and Nott2008; Grant et al. Reference Grant, Ryall, Lyons and Abou-Zaid2010, Reference Grant, Poland, Ciaramitaro, Lyons and Jones2011; Silk et al. Reference Silk, Ryall, Mayo, Lemay, Grant and Crook2011a; Crook et al. Reference Crook, Böröczky, Zylstra, Mastro and Tumlinson2012) and bark sesquiterpenes (Poland and McCullough Reference Poland and McCullough2006; Crook et al. Reference Crook, Krimian, Francese, Fraser, Poland and Sawyer2008; Crook and Mastro Reference Crook and Mastro2010; Grant et al. Reference Grant, Ryall, Lyons and Abou-Zaid2010; Cossé et al. Reference Cossé, Bartelt, Zikowski and Fraser2008; Khrimian et al. Reference Khrimian, Cossé and Crook2011). Of the green leaf volatiles, one compound in particular, (3Z)-hexen-1-ol, is highly antennally active and attractive to males in green prism traps (de Groot et al. Reference de Groot, Grant, Poland, Scharbach, Buchan and Nott2008; Grant et al. Reference Grant, Ryall, Lyons and Abou-Zaid2010; Silk et al. Reference Silk, Ryall, Mayo, Lemay, Grant and Crook2011a). Results of these Canadian studies demonstrate that specific host volatiles are important kairomones used by emerald ash borers for host location, and are also useful as trap lures for surveys of early detection.
Early detection is critical to ash management, and the discovery of a female-produced sex pheromone, the macrocylic lactone, 3Z-dodecen-12-olide (3Z-lactone) (Bartelt et al. Reference Bartelt, Cosse, Zilkowski and Fraser2007; Silk et al. Reference Silk, Ryall, Mayo, Lemay, Grant and Crook2011a), has provided a more sensitive detection tool for monitoring at low-population levels. It appears to operate at short range as shown by sensory deprivation experiments (Pureswaran and Poland Reference Pureswaran and Poland2009). The activity of this female-produced lactone pheromone is synergised by the host kairomone, (3Z)-hexen-1-ol. Canadian studies have shown that this synergism can be exploited by placement of baited green, sticky, prism traps in the tree canopy where mating occurs (de Groot et al. Reference de Groot, Grant, Poland, Scharbach, Buchan and Nott2008; Grant et al. Reference Grant, Ryall, Lyons and Abou-Zaid2010) also, addition of (3Z)-lactone to traps significantly increases detection of emerald ash borers at both the trap and plot level, compared with the standard traps baited with (3Z)-hexenol alone (88% versus 60%, respectively) (Ryall et al. Reference Ryall, Fidgen, Silk and Scarr2012).
The contact sex pheromone of emerald ash borer has been identified as the cuticular hydrocarbon, 9-methylpentacosane (Silk et al. Reference Silk, Ryall, Lyons, Sweeney and Wu2009). It appears only on the cuticle of female A. planipennis at sexual maturity (7–10 days old) and stimulates full copulatory activity in males upon antennal contact; 3-methyltricosane may also be involved as an additional component but shows much weaker activity (Lelito et al. Reference Lelito, Boeroeczky, Jones, Fraser, Mastro and Tumlinson2009).
The European woodwasp
The detection of the European woodwasp, Sirex noctilio Fabricius (Hymenoptera: Siricidae), in Canada and the United States of America (Ciesla Reference Ciesla2003; Hoebeke et al. Reference Hoebeke, Haugen and Haack2005) highlighted an urgent need to develop a trapping system so that the distribution and density of the wasp can be monitored effectively. Introduction of the European woodwasp in other countries has already resulted in one of the most damaging biological invasions of pine forests in the southern hemisphere and poses a serious threat to pine monocultures (Pinus) in both the United States of America and Canada (de Groot et al. Reference de Groot, Nystrom and Scarr2006). Although the risk to pine resources in natural northern forests (mixed coniferous and deciduous) is difficult to estimate, the risk to the commercial pines alone is likely considerable.
Several studies have shown that stressed pine trees are primarily attacked by the European woodwasp, apparently attracted by volatiles, which are released from the stem (Simpson Reference Simpson1976). The volatile composition of pine trees and the antennal responses they elicit in the European woodwasp have been studied in some detail by American researchers (Crook et al. Reference Crook, Böröczky, Zylstra, Mastro and Tumlinson2012). Despite the testing of several promising terpene blends, as well as other treatments, there is still no effective lure that is as attractive as a Lindgren funnel or IPM Tech intercept panel trap placed on trees stressed by trunk-injection with herbicide. A mixture of α-pinene (70%) and β-pinene (30%) is used as a terpene blend for trapping in the United States of America at the present time.
The potential for pheromone-based sexual communication of S. noctilio has been examined by American researchers through fractionation of wasp body washes, followed by male copulatory response assays. This approach has led to the identification of three contact sex pheromone components, (Z)-7-heptacosene, (Z)-7-nonacosene, and (Z)-9-nonacosene (Böröczky et al. Reference Böröczky, Crook, Jones, Kenny, Zylstra and Mastro2009). There is currently little evidence that the European woodwasp uses long-range sex pheromone communication, although Cooperband et al. (Reference Cooperband, Boeroeczky, Hartness, Jones, Zylstra and Tumlinson2012) identified a potential male-produced pheromone candidate, but no field trapping data are available. This compound, (3Z)-decen-1-ol, may, in fact, be a volatile male-produced lek signalling pheromone (Thompson et al. Reference Thompson, Grebenok, Behmer and Gruner2013). The natural history of siricid woodwasps is a complex interaction among three organisms: the wasp, a symbiotic wood-rotting fungus, and the host tree. The symbiotic fungus Amylostereum areolatum (Chaillet) Boidin (Amylostereaceae) is essential for growth and development of larvae of the European woodwasp. In addition, the role of esterified sterols in Sirex physiology needs further investigation, and the potential in defining symbiotic associations and pheromone signalling is possibly a fruitful area of research.
The Asian longhorned beetle
The Asian longhorned beetle, Anoplophora glabripennis (Motschulsky) (Coleoptera: Cerambycidae: Lamiinae), is a serious pest of hardwood trees in Asia, North America, and Europe (Haack et al. Reference Haack, Hérard, Sun and Turgeon2010). The Asian longhorned beetle attacks living trees, preferring maple Acer Linnaeus and horse chestnut, Aesculus hippocastanum Linnaeus (Sapindaceae) in the United States of America, but other hardwood species are also attacked (Haack et al. Reference Haack, Hérard, Sun and Turgeon2010). The Asian longhorned beetle has enormous destructive potential as it spends much of its life as a grub inside tree trunks and branches causing severe damage and tree death. Few natural enemies have been identified, and only soil or tree injection, and bark spray of insecticides are effective. These control tactics are only viable in urban forestry situations, and widespread eradication efforts are presently being conducted using tree removal, at the cost of billions of dollars. Currently, detection of the Asian longhorned beetle relies on visual inspection for signs of emergence from host trees; thus, efficient monitoring traps are needed to detect populations at ports of entry and to assess population density and dispersal.
Several recent attempts to develop attractants for detection and monitoring of Asian longhorned beetle have produced positive results, but these semiochemicals remain to be tested in Canada. Two male-specific volatiles were found to elicit strong electrophysiological and olfactometric responses from both sexes. These volatiles consist of a 1:1 blend of functionalised dialkyl ethers, 4-(n-heptyloxy) butanal and 4-(n-heptyloxy) butan-1-ol (Zhang et al. Reference Zhang, Oliver, Aldrich, Wang and Mastro2002). Nehme et al. (Reference Nehme, Keena, Zhang, Baker, Xu and Hoover2010) trapped Asian longhorned beetles with pheromone alone or in combination with plant volatiles. Traps baited with the male-produced pheromone alone caught significantly more females than control traps but only in low numbers. The addition of a mixture of (–)-linalool, (Z3)-hexen-1-ol, linalool oxide, trans-caryophyllene, and trans-pinocarveol to the pheromone significantly increased capture of virgin females. Synergism between pheromone and host kairomones appears important, and further work in this direction would be fruitful. Cuticular hydrocarbon extracts of female Asian longhorned beetles contain five alkenes: (Z9)-tricosene, (Z9)-pentacosene, (Z7)-pentacosene, (Z9)-heptacosene, and (Z7)-heptacosene, which together elicit copulatory behaviour in males (Zhang et al. Reference Zhang, Oliver, Chauhan, Zhao, Xia and Xu2003). There is some evidence that females autooxidise these cuticular olefins to produce antennally active aldehydes (heptanal, nonanal, hexadecanal). Traps baited with blends of these aldehydes combined with linalool oxide and other host kairomones captured more male beetles than unbaited control traps but only low numbers of beetles were captured (Wickham et al. Reference Wickham, Xu and Teale2012).
In an attempt to use insecticide-treated sentinel trees to monitor the Asian longhorned beetle, five tree genera known to be hosts of the beetle in China were tested for their attractiveness to the beetles. Of the five trees tested, Acer mono Maximowicz was the most attractive and it was significantly more attractive than Acer platanoides Linnaeus, the commonly attacked maple species in the United States of America (Smith et al. Reference Smith, Wu, He, Xu, Gries and Gries2007). Female Asian longhorned beetles were more attracted to girdled sentinel A. mono trees than intact trees, suggesting a role for plant kairomones in host location in this species (Smith et al. Reference Smith, Wu, He, Xu, Gries and Gries2007).
Asian longhorned beetles were first detected in 2003 in Toronto, Ontartio, Canada and neighbouring Vaughan. The federal (Canadian Food Inspection Agency) and provincial governments established a “regulated area” to prevent the spread of the insect, taking down 30 000 trees and banning the movement of trees, logs, wood, and bark chips from susceptible species throughout the area. As of 2014, the beetle has never been detected anywhere in Canada outside the regulated area. The development of pheromone tools for this insect will aid in the detection of future invasions into Canada.
The banded elm bark beetle
The banded elm bark beetle, Scolytus schevyrewi Semenov (Coleoptera: Curculionidae: Scolytinae), is an exotic invasive bark beetle native to China and Russia and was first reported in the United States of America in 2003 in elms in Colorado. Although not confirmed, there is concern that the banded elm bark beetle may be a vector of the Dutch elm disease fungus Ophiostoma novo-ulmi Brasier (Ophiostomatoceae). The host range, chemical ecology, and impact of the banded elm bark beetle have been studied in the United States of America (Negroin et al. Reference Negroin, Witcosky, Cain, LaBonte, Duerr and McElwey2005; LaBonte Reference LaBonte2010; Lee et al. Reference Lee, Negroin, McElwey, Williams, Witcosky and Popp2011), and it is anticipated that this insect will have a significant economic impact on the urban and forested landscapes of North America in the near future.
Lindgren multi funnel-trap catches in Colorado and Nevada, United States of America (Lee et al. Reference Lee, Hamud, Negron, Witcosky and Seybold2010) showed that banded elm bark beetles respond to Multilure, a commercial lure for Scolytus multistriatus Marsham (Coleoptera: Curculionidae), the European elm bark beetle. This lure contains mixtures of 2-methy-3-buten-2-ol and mutistriatin [(1S,2R,4S,5R)-(–)-2,4-dimethyl-5-ethyl-6,8-dioxabicyclo[3.2.1]octane] (Pearce et al. Reference Pearce, Gore, Silverstein, Peacock, Cuthbert and Lanier1975) and a plant extract. The European elm bark beetle responds much more strongly to this blend than the banded elm bark beetle in trapping assays; both Scolytus Geoffroy species give good electrophysiological responses to 2-methy-3-buten-2-ol, (+)- and (−)- α- pinene and racemic multistratin (Lee et al. Reference Lee, Hamud, Negron, Witcosky and Seybold2010). The flight responses of the banded elm bark beetle to plant extracts from Ulmus pumila Linnaeus (Ulmaceae) are described by Lee et al. (Reference Lee, Hamud, Negron, Witcosky and Seybold2010). Antennal response to (–)-α- pinene is greater in the banded compared to European elm bark beetle; the role of this compound as part of host selection behaviour needs to be explored to potentially help improve the trap bait. The banded elm bark beetle was detected for the first time in Canada near Kelowna, British Columbia in 2010. Fifty-eight beetles were incidentally captured in a set of trapping experiments that targeted the European elm bark beetle (Humble et al. Reference Humble, Smith, Zilahi-Balogh, Kimoto and Noseworthy2010).
Conclusions and synthesis
Canadian research has advanced semiochemical-based management of forest pests in Canada and around the world. Research has spanned basic questions on signal identification and chemical ecology, to applied management questions emphasising practicality and affordability for the end user. In many instances, semiochemicals have become indispensible tools in forest pest management. The recent registration of a high-dose formulation of verbenone for protection of pine trees from the mountain pine beetle (Borden et al. Reference Borden, Sparrow and Gervan2007) and a sprayable mating disruption formulation for the spruce budworm (reviewed in Rhainds et al. Reference Rhainds, Kettela and Silk2012) signal receptivity by industrial and government stakeholders of semiochemical-based management tactics. Canadian research has formed the foundation upon which future work can build and hopefully expand the suite of semiochemical-based tools that are operationally available to the forest pest manager.
There is good evidence that climate change has already and will continue to influence the distribution and size of forest insect populations and their hosts. A warming climate is believed to be one of the main drivers in the recent range expansion of the mountain pine beetle (Carroll et al. Reference Carroll, Taylor, Régnière and Safranyik2004) that has now reached jack pine on the western edge of the boreal forest (Cullingham et al. Reference Cullingham, Cooke, Dang, Davis, Cooke and Coltman2011). Recent research suggests that the interaction between the mountain pine beetle and its hosts in the expanded range may differ compared with what is known from its historical range (Clark et al. Reference Clark, Carroll and Huber2010; Cudmore et al. Reference Cudmore, Björklund, Carroll and Lindgren2010). As this interaction is mediated by semiochemicals, future research should examine how new pine hosts and environmental conditions (Lusebrink et al. Reference Lusebrink, Evenden, Blanchet, Cooke and Erbilgin2011, Reference Lusebrink, Erbilgin and Evenden2013) may influence chemically-mediated interactions and ultimately semiochemical-based management in the expanded range of the beetle.
Climate change and global travel will result in the continued arrival of invasive insect species that may be able to exploit and become established in Canadian forest ecosystems. Canada will need expertise in the taxonomy of insects to identify the invaders as well as chemical ecologists who can identify, synthesise, and apply semiochemical signals to monitor invasive insects that pose a threat to Canada’s forest resources and ecosystems. Semiochemical-based monitoring is the only efficient way to monitor introduced species that are typically at low densities at the beginning of the invasion process. Semiochemicals also present an environmentally sound approach to manage invasive species, if they do become established. In the late 1980s, heightened interest in developing ecologically safe methods for managing gypsy moth populations led to the initiation of a mandated Appalachian IPM gypsy moth project in Virginia, with the major goal being to apply mating disruption technology for “slowing the spread” and reducing the impact of gypsy moth populations (Reardon et al. Reference Reardon, Leonard, Mastro, Leonhardt, McLane and Talley1998). This was followed in 1993 by the United States Department of Agriculture Forest Service Slow-the-Spread campaign using Hercon-laminated pheromone flakes. These programmes resulted in a successful reduction in the spread of gypsy moth by 35% from 1988 to 1994 (Sharov et al. Reference Sharov, Liebhold and Roberts1996) as measured by an elegant modelling of the process (Sharov and Liebhold Reference Sharov and Liebhold1998). This approach to gypsy moth control has not been adopted for established populations in eastern Canada and should be considered as a proven pest management tactic in the future. Mating disruption can be a successful tool to slow the spread of other recently established invasive pests such as brown spruce longhorned beetle (P.J.S., personal observation) or to limit population growth of native forest defoliators (Rhainds et al. Reference Rhainds, Kettela and Silk2012) that undergo cyclical changes in population density. This semiochemical-based tactic is currently underused in IPM of forest pests in Canada.
Current research on insect chemical ecology in Canada has delved into understanding geographic and genetic variation in chemically mediated interactions that dictate important life-history events. There are several cases in the literature in which geographic and host-associated variation of chemical communication have been identified in insects. In Canada, the sex pheromone signals of the eastern and western hemlock loopers are distinct (Gries et al. Reference Gries, Bowers, Borden, Underhill, West and Slessor1993a; Li et al. Reference Li, Bikic, Slessor, Gries and Gries1993a, Reference Li, King, Bowers, West, Gries and Gries1993b). Populations of the transcontinentally distributed fir coneworm, Dioryctria abietivorella (Grote) (Lepidoptera: Pyralidae) have different pheromone blends (Grant et al. Reference Grant, Trudel and Millar2009) that dictate the use of different semiochemical-based monitoring tools in eastern and western Canada (Strong et al. Reference Strong, Millar, Grant, Moreira, Chong and Rudolph2008). Recent Canadian research has resulted in the publication of the mountain pine beetle genome (Keeling et al. Reference Keeling, Yuen, Liao, Docking, Chan and Taylor2013), which will assist researchers in discovering genes that are important for the survival of the beetle in its historical and expanded range. Recent international collaborative efforts involving Canadian scientists (Andersson et al. Reference Andersson, Grosse-Wilde, Keeling, Bengtsson, Yuen and Li2013) have identified protein families important for chemoreception in the mountain pine beetle that could be targets of pest management tactics in the future.
Despite advances in chemical ecology research and applications of semiochemicals to forest pest management, there remain forest insect pests for which the discovery of important semiochemicals remains illusive. Future Canadian research could incorporate strategies such as “reverse chemical ecology” in which important chemical signals may be discovered after odorant binding proteins are characterised using molecular techniques. The odorant receptors can be expressed and tested with a variety of ligands to uncover potential important semiochemicals (Witzgall et al. Reference Witzgall, Cork and Kirsch2010). New techniques in combination with established, traditional chemical ecology approaches will continue to result in the discovery of semiochemicals of native and invasive forest pests in Canada. With the support of government and industrial stakeholders, semiochemical-based management of forest pests will continue to play a large role in forest IPM in Canada.
Acknowledgements
The authors thank Drs. Alfaro, Borden, Langor, and Lindgren for their thorough reviews and useful suggestions on former versions of this manuscript. This work was possible due to funding and support of the Evenden laboratory by the University of Alberta, the Natural Sciences and Engineering Research Council of Canada and to the Silk laboratory from the Canadian Forest Service.







