An egg is equipped to support life, with a profile of essential micronutrients that is unparalleled by any other food. Eggs are relatively low in energy (326·35 kJ (78 kcal)/medium egg) and saturated fat (1·7 g/medium egg) in comparison with other animal products, and can boast the highest quality of dietary protein, with certain dairy foods( Reference Benelam, Roe and Pinchen 1 ). Eggs also represent the principal source of dietary cholesterol, which has been a major barrier to egg consumption, primarily because of the outdated, but popular misconception that egg cholesterol converts directly into serum cholesterol, and thus increased risk of CVD. While dietary and serum cholesterol are the same chemical entity, the concentration of serum cholesterol (LDL-cholesterol) falls under the physiological control of whole-body cholesterol homeostasis. This process can be influenced by numerous dietary factors, including dietary cholesterol and fatty acids, but depends ultimately on the essential requirement of cells for cholesterol, and reciprocal interplay between the biosynthesis of cholesterol in tissues, and absorption of cholesterol and bile acids in the gut.
A growing body of evidence from prospective cohort studies( Reference Qureshi, Suri and Ahmed 2 – Reference Scrafford, Tran and Barraj 4 ), systematic reviews and meta-analyses( Reference Hu, Stampfer and Rimm 5 – Reference Rong, Chen and Zhu 8 ) have culminated in a consensus that eggs, and dietary cholesterol derived from eggs, exert a relatively small and clinically insignificant effect on serum LDL-cholesterol in comparison with other lifestyle factors. In one study, modifiable lifestyle factors accounted for <40 % of CHD mortality, to which eating one egg a day contributed <1 %( Reference Barraj, Tran and Mink 3 ). In 2009, this prompted a relaxation of the long-standing advice in the UK to limit the intake of eggs as the chief source of cholesterol, to no more than three per week( Reference Gray and Griffin 9 ). This action was later endorsed by the American Heart Association, who removed their guideline to restrict dietary cholesterol intake in healthy individuals to 300 mg/d in 2013( Reference Millen, Wolongevicz and de Jesus 10 ), and by the US Dietary Guidelines Advisory Committee in 2015, on the grounds of there being insufficient evidence to link dietary cholesterol with CHD( 11 ).
In spite of these changes, reports of associations between egg consumption and the incidence of diabetes, and increased risk of CVD in diabetes still prevail( Reference Qureshi, Suri and Ahmed 2 , Reference Hu, Stampfer and Rimm 5 , Reference Shin, Xun and Nakamura 7 , Reference Rong, Chen and Zhu 8 , Reference Tanasescu, Cho and Manson 12 – Reference Djoussé, Khawaja and Gaziano 16 ). This may be explained, in part, by residual confounding, with eggs acting as a marker of diets that are higher in saturated fat and energy, and also because eggs are simply easier to quantify than other dietary constituents in methods that rely on 24 h recall. While recent prospective cohort studies have produced evidence to refute the recurring association between eggs and the incidence of type-2 diabetes( Reference Zazpe, Beunza and Bes-Rastrollo 17 – Reference Djoussé, Petrone and Hickson 20 ), a greater understanding of the metabolic response to eggs and dietary cholesterol in different forms of diabetes will be necessary to establish the clinical significance of the association between egg intake and CVD risk in this disease.
Egg: nutrient composition
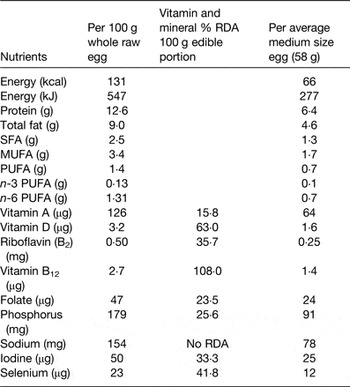
The nutrient to energy density ratio in an egg is high in comparison with most other foods (Table 1)( Reference Benelam, Roe and Pinchen 1 ). A medium-sized boiled egg (58 g) contains 227 kJ (66 kcal) energy (70% of the energy in one medium slice of white bread), 4·6 g fat, of which 2·4 g is unsaturated and 1·3 g is saturated. This represents approximately 11 % of the saturated fat in a typical sausage roll or quarter pounder beef-burger (12 g SFA/100g). The amino acid profile of egg protein (approximately 6·5 g/egg) is similar to that of beef steak, but has greater bioavailability, as indicated by its higher protein digestibility corrected amino acid score (egg 118, beef steak 92, milk 121, soya 9, wheat 42)( Reference Schaafsma 21 ). Dietary protein has long been associated with increased satiety, and there is evidence to suggest that the high protein content of eggs may contribute to this effect( Reference Van der Wal, Marth and Khosla 22 , Reference Fallaize, Wilson and Gray 23 ). Eggs have also been shown to promote weight loss in overweight and obese subjects by increasing feelings of satiety and reducing short-term energy intake( Reference Van der Wal, Gupta and Khosla 24 ). With respect to micronutrients, it is interesting to note that eggs in the UK have been shown to contain higher concentrations of vitamins D3, K and selenium than previously reported( Reference Benelam, Roe and Pinchen 1 , 25 ). This may reflect an increased absorption of lipid-soluble vitamins and nutrients by hens in response to a change in feeding practices, with meat and bone meal being replaced with vegetable oils and other enhancements( Reference Benelam, Roe and Pinchen 1 ). Finally, eggs are well renowned for being the principal source of dietary cholesterol (200 mg/whole medium egg (58 g)), next to certain shellfish( Reference Isherwood, Wong and Jones 26 ).
Table 1. Nutrient composition of eggs( Reference Benelam, Roe and Pinchen 1 )

Historical perspective of the relationship between eggs, dietary cholesterol and CVD
In 1952, Ancel Keys wrote; ‘Arguments that the amount of cholesterol in the diet may be reflected in the blood level are not supported by more critical studies of man. Only at extremely high intakes, far above the cholesterol content of all ordinary diets, is there reason to believe that dietary cholesterol has any influence on blood level’( Reference Keys 27 ). This statement originates from Key's own observations of the relationship between diet and blood cholesterol in populations, and from early metabolic ward studies in the USA, which to this day, still provides some of the strongest evidence for the effects of dietary cholesterol, and exchanging saturated fat with poly and mono-unsaturated fat, on serum cholesterol( Reference Clarke, Frost and Collins 28 ). The prophetic accuracy of Key's observations seems all the more remarkable when you consider that LDL was only discovered and first implicated in coronary disease in the late 1940s( Reference Gofman and Lindgren 29 ), when nutritional epidemiology was in its infancy. There are many reasons why it has taken over 60 years to confirm these observations, one of which has been an inability to discriminate adequately between the influence of dietary cholesterol and saturated fat on serum cholesterol and CVD risk.
The relationship between dietary cholesterol and CVD is similar to that for certain SFA and CVD, in being mediated primarily through an elevation of serum LDL-cholesterol. Although dietary cholesterol and saturated fat are inextricably linked through their consumption in similar foods, the relationship between saturated fat and CVD is more complex than dietary cholesterol, as the former exists as a group of fatty acids of variable chain length and LDL-raising properties( Reference Griffin, Cunnane, Gibney, Lanham-New, Cassidy and Vorster 30 ). Dietary cholesterol is also derived from a limited number of sources, chiefly eggs and not from a wide variety of foods, the other constituents of which have been shown to modify the bioavailability, and thus LDL-raising properties, of the SFA they contain( Reference Hjerpsted, Leedo and Tholstrup 31 , Reference Soerensen, Thorning and Astrup 32 ). Other common confounding factors that have led to spurious associations between eggs, dietary cholesterol and CVD in prospective cohort studies, include the study groups being too homogeneous and unrepresentative of mixed populations (e.g. nurses and physicians), an inadequacy or complete lack of data on dietary intake and other relevant biomarkers of CVD risk, and misleading stratification of egg intake, with a moderate egg intake (seven eggs/week) being grouped with extreme intakes( Reference Gray and Griffin 9 ). Intervention studies that controlled for many of these confounding factors, and especially the intake of saturated fat, found, in the main, no significant effect of egg intake on serum LDL-cholesterol( Reference Edington, Geekie and Carter 33 – Reference Griffin and Lichtenstein 38 ), but clear evidence of a dose–response relationship between dietary cholesterol and serum LDL-cholesterol( Reference Hopkins 34 ), and considerable variation in the response of serum LDL-cholesterol in both men and women( Reference Ginsberg, Karmally and Siddiqui 35 , Reference Ginsberg, Karmally and Siddiqui 36 ). Cross-sectional and prospective cohort studies and meta-analyses, have also reported associations between egg intake and an increased incidence of diabetes, and elevated CVD risk in diabetes( Reference Tanasescu, Cho and Manson 12 – Reference Djoussé, Khawaja and Gaziano 16 ).
Eggs intake, incidence of diabetes and increased CVD risk in diabetes
Diabetes is a collection of diseases of variable aetiology and metabolic characteristics( 39 ). Type-2 diabetes is characterised by tissues being resistant to the actions of insulin and variable degree of insulin deficiency, and typically a late onset. In contrast, type-1 diabetes tends to have an early onset, and is characterised by a failure of insulin production because of the destruction of β-islet cells in the pancreas. Diabetes has been described as being a risk factor for CVD in itself, and thus independent of the adverse effects of raised serum LDL-cholesterol. Nevertheless, it is conceivable that metabolic differences in the handling of dietary cholesterol between types 1 and 2 diabetes underlies the variable response of serum LDL-cholesterol to egg-derived cholesterol in these groups.
Evidence for associations between egg intake and increased incidence of diabetes in meta-analyses of prospective cohort studies has been inconsistent, some of which support associations( Reference Tanasescu, Cho and Manson 12 – Reference Djoussé, Khawaja and Gaziano 16 ), while others do not( Reference Zazpe, Beunza and Bes-Rastrollo 17 – Reference Djoussé, Petrone and Hickson 20 ). Egg intake is a well known marker for the intake of dietary fat and energy, both of which can contribute to an increase in body weight and risk of developing diabetes. In addition, studies in diabetes have also been confounded by the disease being self-reported and not medically diagnosed, small number of cases, and major inconsistencies in the exclusion and inclusion criteria of patients and their medication, including insulin. All of these factors were identified as potential confounders in a recent systematic review by Tran et al. ( Reference Tran, Barraj and Heilman 40 ), which concluded that there was no consistency in the findings for an association between egg intake and the incidence of type-2 diabetes. Further support for this conclusion comes from the outcome of prospective cohorts in the Mediterranean, Finland, Japan and North America( Reference Zazpe, Beunza and Bes-Rastrollo 17 – Reference Djoussé, Petrone and Hickson 20 ), all of which found no significant association between egg consumption and the incidence of type-2 diabetes. The North American Jackson Heart Study did show a significantly higher prevalence of type-2 diabetes cross-sectional, but no prospective link between egg intake and the development of diabetes( Reference Djoussé, Petrone and Hickson 20 ). Interestingly, when the Jackson Heart Study cohort was stratified according to egg intake (<1 egg/month to 5+/week), higher egg consumption was significantly associated with greater amounts of saturated and trans fatty acids, red meat and food energy than the low egg consumers (P < 0·0001 for all comparisons). This reinforces the evidence for the confounding nature of the dietary factors in producing a false-positive association between egg intake and CVD risk in diabetes. It also supports the outcome of a recent study of dietary patterns that attributed associations between egg intake and CVD risk factors (body mass and waist circumference), to a small number of distinct dietary patterns that included eggs, thus implicating the contribution of other dietary components to this association( Reference Nicklas, O'Neil and Fulgoni 41 ).
Impact of egg feeding on serum LDL-cholesterol in diabetes
In comparison with the evidence to support associations between eggs and CVD in diabetes, there is relatively little evidence for the effects of egg interventions on serum LDL-cholesterol. An extremely high intake of egg-derived cholesterol (800 mg/d, 3–4 eggs/d) was shown to increase serum LDL-cholesterol in patients with type-1 diabetes, but not in type-2 diabetes( Reference Romano, Tilly-Kiesi and Patti 42 ), a finding that has never been substantiated for lower, more physiological intakes of eggs in these different patient groups. A lack of effect of eggs on serum LDL-cholesterol in type-2 diabetes was reported in response to a high protein, energy-restricted diet that resulted in significant reductions in body weight, total serum cholesterol, non-HDL cholesterol and indices of glycaemic control after 12 weeks( Reference Pearce, Clifton and Noakes 43 ), and also in the DIADEGG study, the largest egg intervention study to date in type-2 diabetes( Reference Fuller, Caterson and Sainsbury 44 ). This study tested the effects of high egg diet (2 eggs/d for 6 d for 12 weeks) against a low egg diet (<2 egg/week), and showed no significant effects on body weight or biomarkers of CVD, including serum LDL-cholesterol. Egg-feeding has also been associated with favourable effects on HDL( Reference Andersen, Blesso and Lee 45 ), LDL particle size( Reference Mutungi, Waters and Ratliff 46 ), and increased insulin sensitivity( Reference Blesso, Andersen and Barona 47 ), findings that suggest that eggs may actually reduce the risk of developing type-2 diabetes, and thus CVD, when included as part of a broader dietary intervention( Reference Tran, Barraj and Heilman 40 ). On the contrary, there are still examples of advice that contradict this view. One such example, in a recent editorial in the American Journal of Clinical Nutrition, claimed that eggs should be avoided if you have type-2 diabetes, because the absorption of dietary cholesterol in the gut is increased in this condition( Reference Eckel 48 ). The meta-analysis that was the subject of the editorial that issued this advice concluded that its data were insufficiently robust to absolve eggs from their association with CVD, but it did not study diabetes( Reference Berger, Raman and Vishwanathan 49 ). Moreover, the evidence to support an increase in cholesterol absorption in type-2 diabetes was restricted to the outcome of a single gene expression study( Reference Lally, Tan and Owens 50 ). It ignored data from metabolic studies that showed cholesterol absorption to be lower, and cholesterol synthesis to be higher, in type-2 diabetes than non-diabetic controls, when measured in vivo using stable isotope tracers, and plant sterols as biomarkers of cholesterol synthesis and absorption( Reference Gylling and Miettinen 51 ). The latter techniques would be useful to investigate the origin of the variable serum LDL-response to egg-derived cholesterol in different types of diabetes.
Variation in metabolic response to eggs and dietary cholesterol
Human subjects have an essential requirement for cholesterol as a component of cell membranes and for the production of plasma lipoproteins, steroid hormones, vitamins and bile acids that facilitate fat digestion. For this purpose, cells have an innate capacity to sense their own requirement for cholesterol by detecting the amounts of intracellular free cholesterol, and adjusting their rates of cholesterol biosynthesis and/or uptake of LDL-cholesterol from the serum through the production of LDL receptors on their cell surface. Intracellular free cholesterol is also influenced by the rate of absorption of cholesterol (dietary and biliary) and bile acids in the gut, which is inversely related to cholesterol biosynthesis and uptake( Reference Griffin, Cunnane, Gibney, Lanham-New, Cassidy and Vorster 30 ). The metabolic efficiency within, and flexibility of the interplay between, these pathways can be influenced by genetic and environmental factors, and may help to explain variation in the LDL-cholesterol response to dietary cholesterol. For example, increasing dietary cholesterol will lead to increased absorption and increased intracellular free cholesterol, which should in turn, down-regulate biosynthesis and uptake of LDL from serum, resulting in a rise in serum LDL-cholesterol. In other words, the more or less cholesterol that is absorbed from the gut, the less or more cholesterol cells acquire, through biosynthesis or from circulating serum LDL. However, there is evidence to suggest that the sensitivity of this reciprocal mechanism, in terms of the ability to down-regulate cholesterol absorption above a certain threshold of dietary intake, is highly variable( Reference Ostlund, Bosner and Stenson 52 ). Nevertheless, it is still possible to categorise people into those with high rates of cholesterol synthesis (low absorbers) or high rates of cholesterol absorption (low synthesisers)( Reference Sehayek, Heinemann and McGee 53 ), a phenomenon that is known to contribute to the differential response to cholesterol-lowering drugs that target either cholesterol synthesis (statins) or absorption (ezetimibe)( Reference Telford, Sutherland and Edwards 54 ). Variation in the capacity to make metabolic adjustments, particularly in down-regulating cholesterol absorption, and the background metabolic phenotype (predominant ‘absorber’ or ‘synthesiser’ of cholesterol), may all contribute to the variability in serum LDL-cholesterol response to egg-derived cholesterol, and the risk association between CVD and diabetes, and different types of diabetes.
Influence of metabolic dysfunction in diabetes on the serum LDL-cholesterol response to eggs and dietary cholesterol
The metabolic characteristics of types 1 and 2 diabetes may give rise to distinct differences in the way in which egg-derived cholesterol is handled. Insulin resistance seems to be inversely associated with the absorption of cholesterol in the intestine( Reference Gylling, Hallikainen and Pihlajamäki 55 , Reference Knopp, Retzlaff and Fish 56 ), which in terms of an influence on serum LDL-cholesterol, should render type-2 diabetes less, rather than more sensitive to egg-derived cholesterol. While this would be consistent with the lack of effect of egg-feeding on serum LDL-cholesterol in type-2 diabetes, the same may not apply to type-1 diabetes. The permeability of the intestinal epithelium has been shown to increase in diabetes, which could impact on the mechanisms that regulate cholesterol absorption( Reference Horton, Wright and Smith 57 ). Whether this phenomenon is differentially expressed in different types of diabetes is currently unknown. Patients with type-1 diabetes can also develop an autonomic neuropathy in the intestine that is closely linked to CVD, and can delay gastric emptying, increase whole gut transit time and reduce contraction of the gall bladder( Reference Werth, Meyer-Wyss and Spinas 58 , Reference Wegener, Börsch and Schaffstein 59 ). While this is an understudied condition in this type of diabetes, it has major implications for the differential effects of egg-derived cholesterol on serum LDL via dysregulation of the enterohepatic circulation of cholesterol and bile acids, and absorption of dietary cholesterol.
Conclusion
From a nutritional perspective, a moderate intake of eggs is an important component of a balanced pattern of food intake. The micronutrient and protein content of eggs may also have utility in maintaining the nutrient density and efficacy of an energy-restricted diet for the purpose of losing body weight. So, from a nutritional standpoint, eggs must be considered ‘good’. The burden of proof that eggs are not ‘bad’, lies with their exoneration from associations with CVD, diabetes and CVD in diabetes. There is now general consensus that the egg intake at moderate levels of daily intake has no clinically significant effects on CVD risk in the general population, and dietary guidelines have been revised accordingly. Evidence that eggs increase the likelihood of developing diabetes is inconsistent, and the association between eggs and increased CVD risk in diabetes is heavily confounded by a host of dietary and patient-related factors. What has become increasingly apparent is that types 1 and 2 diabetes may respond differently to egg-derived cholesterol, creating the possibility that the association between egg intake and CVD risk may exist for one type of diabetes, but not the other.
Financial Support
None.
Conflicts of Interest
Professor Griffin is a scientific adviser to the British Egg Information Service.
Authorship
The author was solely responsible for all aspects of preparation of this paper.