INTRODUCTION
Campylobacter species are the most frequent bacterial aetiology of gastroenteritis associated with foodborne diseases worldwide [Reference Alteknuse1–Reference Moore3]. Symptoms of campylobacteriosis include diarrhoea, stomach pain and fever [Reference Coker4]. The illness is usually self-limiting and symptoms should subside within 1 week; however, infection may lead to complications such as dehydration, bacteraemia, hepatitis and neurological damage [Reference Wassenaar and Blaser5]. Death is rare but occasionally occurs in young children, the elderly and immunocompromised individuals [Reference Mead6]. Incubation periods range from 2 to 7 days [Reference Evans7, Reference Tsunematsu8] and average shedding is about 1–3 weeks following the incubation period depending on the setting [Reference Calva9–Reference Blaser, Mandell, Bennett and Dolin12]. In humans, C. jejuni and C. coli are the most commonly isolated species of Campylobacter in cases of gastrointestinal diseases [Reference Wasfy13–Reference Said15]. Humans acquire Campylobacter infections chiefly through handling and/or consumption of contaminated food or drinks; mainly poultry, untreated water and unpasteurized milk [Reference Kassa, Gebre-selassie and Asrat14, Reference Tauxe, Nachamkin, Blaser and Tompkins16, Reference Skirrow, Blaser, Blaser, Smith, Ravdin, Greenberg and Guerrant17]. Direct and indirect contact with infected animals (including poultry) and/or their faeces is also a risk factor for acquisition of infection by Campylobacter species [Reference Anderson, Horn and Gilpin18]. In developing countries, Campylobacter infections occur most frequently in children as older children and adults acquire a level of protective immunity following exposure [Reference Calva9, Reference Georges-Courbot19, Reference Blaser20].
Few data exist regarding the epidemiology of Campylobacter species in poultry and children in Egypt. In a survey conducted in Alexandria, Egypt, Campylobacter species were isolated from 16·8% of 880 children suffering from diarrhoea compared to 6·4% of 1079 healthy children [Reference Pazzaglia21]. In a hospital-based study in Cairo, Campylobacter were isolated from 37/143 (25·9%) of diarrhoeic children compared to 20/132 (15·2%) of non-diarrhoeic children (P < 0·05) [Reference Pazzaglia22].
The aim of this study was to investigate whether children exposed to backyard poultry infected with Campylobacter were at higher risk of being infected by Campylobacter compared to those that were exposed to backyard poultry which were Campylobacter negative. Risk factors for backyards being classified as positive were then investigated. To the best of our knowledge this is the first study assessing the risk to children posed by Campylobacter species colonization of backyard poultry.
METHODS
Study design
This study was conducted during April 2011 to May 2012. The study area consisted of four villages located in Gharbia governorate in the Nile Delta region of Egypt, ~100 km north of Cairo (Fig. 1).

Fig. 1 [colour online]. The study area of the Gharbia governorate in the Nile Delta region of Egypt.
The sample size was 100 households; which would be sufficient to detect a difference in proportions with a confidence of 95% and power of 80%, assuming the proportion of households positive for Campylobacter infection was 30% in the exposed group (Campylobacter +ve backyard poultry) compared to 5% of households positive for Campylobacter in the unexposed group. It was decided that four villages would be sampled in order to ensure that any effects observed were not specific to one village. After consultation with the General Authority for Veterinary Services (Gharbia Branch), a suitable list of villages was obtained in rural communities of the governorate where most households raised chickens, ducks and/or turkeys on a small scale to supplement their income. Estimates of the number of households were obtained and sampling proportional to size was used to select four villages; with larger villages having a higher probability of being selected. Households were then selected using systematic sampling.
The target sample size for each village was 25 households; the sampling interval for each village was obtained by dividing the number of households by the target sample size. A road leading from the village centre was randomly sampled and systematic sampling began from this road, the first house sampled was dependent on the sampling interval. Sampling continued either side of the road until the border of the village was reached; and the first road clockwise was taken back towards the village centre. Next, another road opposite to the first road was selected and sampling continued in this manner. Households were sampled if they met the following two criteria: (1) owned between 10 and 100 head of backyard poultry; (2) presence of at least one child aged between 7 and 15 years in frequent contact with live poultry (i.e. involved in feeding or cleaning the backyard). If a household did not meet these criteria they were excluded and a neighbouring household was visited, until a suitable household was found.
Within poultry backyards those with ⩽50 birds had three birds sampled, those with >50 birds had four birds sampled. Birds were selected for sampling using simple random sampling; cloacal swabs were collected from each selected poultry. The date of sampling, location, household ID and poultry species were recorded. All available children who were in contact with live backyard poultry aged between 7 and 15 years in each household were sampled. Children's stool samples were collected in individual sterile containers and the date of sampling, location, household ID and the age and sex of each child were recorded.
Samples from children and backyard poultry were collected on the same day. All samples were stored on ice and transported to the laboratory within 2 h of collection. Bacterial isolation and identification of C. jejuni and C. coli was performed. The laboratory work was performed at El-Slam Laboratory, Tanta, Egypt and the Faculty of Veterinary Medicine–Kafrelsheikh University (FVM-KU) Laboratory. During the sample collection, data on biosecurity and management measures was recorded by observation of the examined backyards and discussions with livestock owners. Data collected included: (1) whether the poultry were housed in similar or different age groups; (2) whether poultry were housed with different poultry species; (3) presence or absence of other livestock in the same poultry backyard; (4) regularity of drinking water sanitation; (5) frequency of feeding sanitation; (6) observations on the cleaning and disinfection of poultry house, surrounding areas and utensils; (7) the litter quality, i.e. was there presence of wet littler; (8) whether the manure disposal was inside or outside the backyard; (9) whether ill birds were isolated from the flock.
Ethical approval was obtained by a committee from the Ministry of Health and Population – Gharbia Directorate, for institutional care and concern, for children, while sampling of poultry was approved by a committee from FVM-KU for institutional bird care and use.
Isolation of Campylobacter species
Campylobacter selective agar (Preston) was used for selective isolation of C. jejuni and C. coli from children and poultry samples. The selective media was prepared from Campylobacter agar base (CM0689, Oxoid Ltd, UK), Preston Campylobacter Selective Supplement (SR0117, Oxoid Ltd, UK) and lysed horse blood (SR0048, Oxoid Ltd, UK). Each cloacal swab or 0·5 g children's stool was emulsified in 2 ml of 0·1% peptone water and then inoculated onto the selective medium by cotton-tipped swab. The inoculated plates were loaded in anaerobic jars (HP0011, Oxoid Ltd, UK) and incubated at 42°C for 24–48 h under microaerophilic conditions. The Oxoid Gas Generating kit for Campylobacter species (BR0056, Oxoid Ltd, UK) and an active catalyst were used to obtain a microaerophillic atmosphere of about 5–6% oxygen, 10% carbon dioxide and 84–85% nitrogen.
Identification of Campylobacter species
The positive control was C. jejuni ATCC® 29428 and the main characteristic features were good growth with grey-brown coloured colonies. The negative control was Escherichia coli ATCC® 25922 with inhibited growth. All colonies exhibiting morphology and motility typical of Campylobacter species, Gram staining and the oxidase test were used for the identification of C. jejuni and C. coli. Biochemical tests, i.e. hippurate hydrolysis and indoxyl actetate hydrolysis, were applied for phenotypic characterization of selected thermophilic Campylobacter species [Reference Zaman23, Reference Wagenaar and Jacobs-Reitsma24]. C. jejuni was positive for hydrolysis of hippurate and indoxyl acetate, whereas C. coli was negative for hydrolysis of hippurate and positive for hydrolysis of indoxyl acetate.
Statistical analysis
All analyses were performed at the household level and C. jejuni and C. coli were analysed separately; presence of at least one positive poultry sample resulted in the backyard being classified as positive for Campylobacter and the contact children considered exposed. The percentage of poultry and children positive for each Campylobacter species was calculated. C. coli infections in children were rare, therefore further analysis focused on C. jejuni.
Initially, analysis was performed using χ 2 tests; any variables associated with outcome with a P value ⩽0·2 were then retained for further analysis. Logistic regression models were used to calculate odds ratios and confidence intervals for the association between households with poultry positive to C. jejuni (exposure) and the presence of children positive to C. jejuni within these households (outcome). Risk factors for the presence of C. jejuni in backyard poultry were then investigated; all variables retained from the first analysis step were included in the logistic regression model in order to control for potential confounding; variables which were associated with the outcome with a P value >0·05 were then removed from the final model. In both models village was included as a fixed effect to control for the correlation of households within villages; and to assess whether there were significant differences in C. jejuni prevalence between villages. Due to collinearity between management practices and limited sample size; only univariate analysis was performed, therefore the results of the current study have not been controlled for confounding. All analyses were performed using Stata v. 11 (StataCorp., USA).
RESULTS
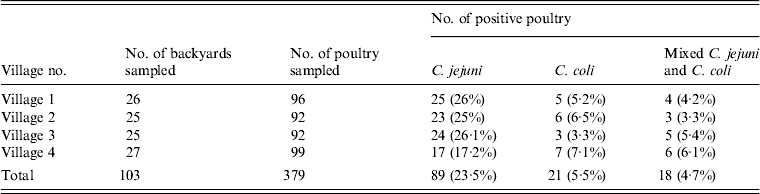
A total of 1383, 1276, 1297 and 1387 poultry were housed in 26, 25, 25 and 27 backyards in the studied villages, respectively. The numbers of poultry (mean ± s.d.) from the examined backyards in villages 1, 2, 3 and 4 were 53.19 ± 22.40, 51.04 ± 22.73, 51.88 ± 22.09 and 51.37 ± 21.00, respectively. Of the 379 birds sampled, C. jejuni, C. coli and mixed infections were present in 89 (23.5%), 21 (5.5%) and 18 (4.7%) birds, respectively (Table 1). This equated to 57 (55.3%) and 25 (24.3%) backyards being classified as positive for C. jejuni and C. coli, respectively. Thirty-five backyards were negative for both species.
Table 1. Percentage of backyard poultry positive for C. jejuni and C. coli
A total of 27, 26, 25 and 28 children were sampled from 26, 25, 25 and 27 households, respectively. C. jejuni, C. coli and C. jejuni + C. coli combined were isolated from 13 (12.3%), three (2.8%) and three (2.8%) children, respectively (Table 2). Campylobacter infection in children and the status of backyards is given in Table 3.
Table 2. Percentage of stool samples positive for C. jejuni and C. coli in children
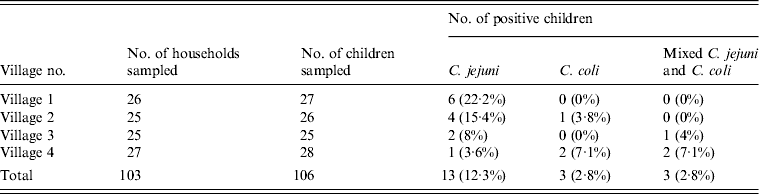
Table 3. Campylobacter infection and exposure status of children
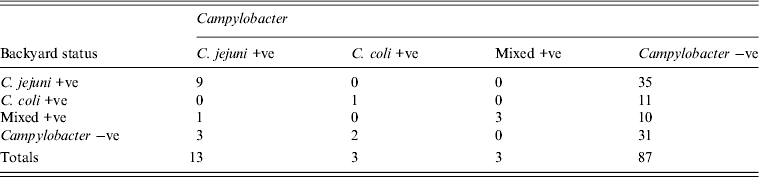
Using logistic regression children from households with at least one bird positive for C. jejuni had 3.86 (95% confidence interval 1.0–15.0) times the odds of being positive for C. jejuni compared to children from households no birds positive. There was no statistically significant association between gender of the child and Campylobacter status (P = 0.42).
Biosecurity and management factors which may be associated with Campylobacter species are presented in Table 4. Mixing species of poultry groups (71·8%) was common as was infrequent feeding sanitation (76·7%), poor cleaning and disinfection (60·2%), presence of wet litter (72·8%), manure inside the backyard (60·2%) and the presence of ill birds inside the backyard (45·6%). Univariate analysis suggested all the above-mentioned management factors were associated with the likelihood of a household being classified as positive for C. jejuni (P < 0·05 for all factors). However, the final multivariable logistic regression model only identified poor cleaning and disinfection, presence of wet litter, and manure inside the backyard as significant risk factors (P < 0·05) (Table 5).
Table 4. Poultry biosecurity and management factors, according to household C. coli and C. jejuni status
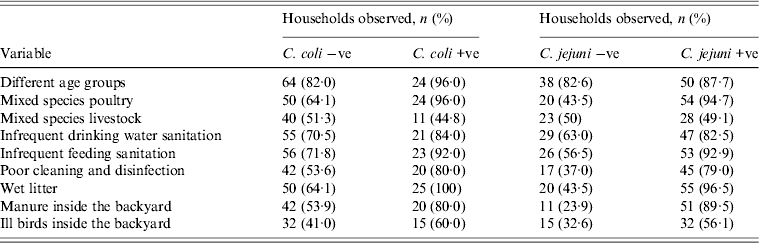
Table 5. Risk factors associated with poultry testing positive for C. jejuni
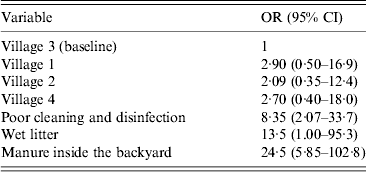
OR, Odds ratio; CI, confidence interval.
DISCUSSION
This is the first study to assess the risk of Campylobacter infection in children exposed to infected backyard poultry in Egypt. Our results indicate that C. jejuni infection is more common than C. coli in both poultry and children in the area. Very limited data are available regarding the prevalence of Campylobacter species in Egypt. A previous study of C. jejuni infection in poultry found isolation rates varied with the organ sampled, with 29% of chicken livers yielding C. jejuni compared to 18·5% from the gizzard, 8·5% from the spleen and 5% from the heart. Higher rates were recorded from chicken giblets (23·5%) compared to duck (19%), turkey (14·5%) and squab (4%) [Reference Khalafalla25].
Previous studies in other countries have also identified C. jejuni as the most common isolate in poultry with C. coli being more frequently isolated from pigs and sheep [Reference Kassa, Gebre-selassie and Asrat14, Reference Jacob, Mdegela and Nonga26–Reference Grove-White29]. Generally, the population of pigs in Egypt is much lower than the poultry populations due to the popular consumption of poultry and the large-scale government culling of around 300 000 pigs during the 2009 H1N1 ‘swine flu’ pandemic [Reference Fahmi and Sutton30].
The current study demonstrates a positive association between the presences of Campylobacter-infected backyard poultry and Campylobacter infection in children. This may represent a potential route of infection. A previous study in Peru using molecular typing (PFGE) suggested that children and poultry from the same household shared similar if not identical strains of C. jejuni suggesting the existence of an infection route from poultry to child [Reference Oberhelman31]. Larger studies including molecular typing and collection of potential confounders, e.g. other livestock, eating habits, other dietary sources, etc. would need to be performed to investigate this potential infection route further in Egypt.
Three children in the current study were infected with both species, as were their backyards. Moreover, C. jejuni was found more frequently than C. coli in backyard poultry and children. Finally, the proportion of obtained isolates was higher in poultry compared to children. Three children were infected with C. jejuni and two with C. coli but these were not isolated from their respective poultry flocks. However, since only four birds were sampled per flock it is likely that there was misclassification with some infected flocks being defined as ‘negative’ on sampling results. Other exposure routes may also account for infection of these children namely ingestion of contaminated water, milk, food and exposure via other livestock or other households' backyard poultry [Reference Oberhelman, Taylor, Nachamkin and Blaser2, Reference Anderson, Horn and Gilpin18, Reference Taylor, Nachamkin, Blaser and Tompkins32, Reference Korlath33].
Our results indicated low biosecurity and management measures in the examined backyards which may enhance the spread of Campylobacter species and increase the risk of exposure to children. Putative risk factors identified included mixing species groups of poultry, poor cleaning and disinfection, high presence of manure and wet litter, presence of ill birds and poor feeding biosafety. However multivariable regression only identified poor cleaning and disinfection, presence of wet litter and manure as risk factors in the present study. Further larger studies in this area are required to identify risk factors and thereby allow development of potential interventions for control.
A previous study found raising of poultry with other species, e.g. cattle and sheep, poor cleaning and disinfection of surrounding area of poultry houses, and presence of manure inside the farm were risk factors for Campylobacter infection in Senegalese broiler flocks [Reference Cardinale34]. The risk of Campylobacter infection may be increase by up to twofold in the presence of wet litter [Reference Berndtson35]. Furthermore; Campylobacter may be found in the water, feed, litter and air of poultry houses [Reference Newell36].
In developing countries many guidelines for good biosecurity in backyard poultry are impractical and have low adoption rates [Reference Conan37]. Information needs to be disseminated to households regarding the risk of Campylobacter to children and methods of reducing exposure, these methods need to be specific, easy to implement and cost-effective, e.g. hand washing, separation of different animal species and ages, isolation of ill birds and improved disinfection practices and the success monitored.
CONCLUSIONS
The findings from this preliminary study indicate that exposure of children to infected backyard poultry may present a route of transmission for Campylobacter infection. Good biosecurity and management must be applied to prevent the transmission of Campylobacter from poultry to children. Increasing public health awareness of households may help to control this zoonotic problem.
ACKNOWLEDGEMENTS
The authors thank Dr Ahmed Eid, Dr Salwa Mohamed and Dr Zaki Saber for their help in collecting the samples.
DECLARATION OF INTEREST
None.









