INTRODUCTION
Cystic echinococcosis (CE) is caused by infections with the larva of the minute tapeworm Echinococcus spp. In Europe, four species are of concern for human CE: E. granulosus s.s. (or ovine strain), E. canadensis, E. equinus, and E. ortleppi (or Swiss strain). Human infection caused by tropical American species E. oligarthrus and E. vogeli is quite anecdotal in Europe
With regard to their life-cycle, adult tapeworms inhabit the small intestine of carnivores (the definitive hosts) and produce eggs, which are passed with faeces. The intermediate host (including sheep, cattle, donkeys, camels) is infected by ingestion of eggs. Subsequently, a larval stage (metacestode) develops as a cyst in the internal organs of the host. The metacestode produces many protoscolices, each with the potential to develop into an adult tapeworm when ingested by the definitive host. Cysts can be either viable or non-viable. Viable cysts are usually filled with clear fluid with few calcifications, whereas non-viable cysts are mainly calcified. Viable cysts can be either fertile, containing protoscolices, or sterile, containing only highly antigenic fluid. People can become intermediate hosts after accidental ingestion of eggs. Developing cysts cause the morbidity and mortality associated with the disease.
CE is still regarded as a neglected disease whose clinical manifestations range from asymptomatic invasion to severe disease with the possibility of death [Reference Bristow1–Reference Belhassen Garcia3]. Although CE is considered to be an eradicable parasite, it has a substantial global disease impact with economic losses for public health systems and agricultural sectors in endemic areas [Reference Budke, Deplazes and Torgerson4]. CE occurs worldwide but it is endemic in central Asia, northern and eastern Africa, Australia, South America and the Mediterranean Basin [Reference Jenkins, Romig and Thompson5–Reference Wahlers7].
Worldwide, animal and human hydatidosis does not appear to have diminished in recent years; in fact, current studies have shown that CE is a re-emerging disease in several countries and regions, even in places where its prevalence was previously low [Reference Jenkins, Romig and Thompson5, Reference Grosso6, Reference Thompson and McManus8]. On the other hand, public health disease control programmes may have decreased the incidence and prevalence of hydatid disease [Reference Gimeno-Ortiz9, Reference Jiménez10]. For instance, various control campaigns have shown that preventive measures against the domestic cycle of E. granulosus may eventually decrease the incidence and prevalence of the disease [Reference Craig and Larrieu11]. Therefore, control campaigns based on health education, control, or elimination of home sheep slaughter have been successfully implemented in five island-based areas (Iceland, New Zealand, Tasmania, Falkland Islands, Cyprus), and two further continental campaigns have been successfully implemented in Latin America (Region XII in Chile, Rio Negro in Argentina). However, several other attempts to control hydatid invasion have failed [Reference Craig12]. This evidence highlights the importance of control programmes for CE disease. The reduction of these programmes due to the lack of economic resources may have catastrophic consequences, leading to severe disease, considerable economic loss and, ultimately, a public health problem of increasing concern [Reference Grosso6].
The transmission rate of E. granulosus in Spain is still high, and is considered highly endemic [Reference Rojo-Vazquez13]. The central, northeastern and western regions of Spain are the most important endemic regions, where extensive or semi-extensive farming of livestock (mostly sheep) is common. In particular, Salamanca province, which is located in the west, presents a higher incidence rate of hydatid disease compared to other Spanish regions [Reference Pardo14]. Since the mid-1980s, several prevention and control programmes have been implemented to reduce E. granulosus infection in Spain [Reference Benner15]. However, lack of epidemiological results prevents the evaluation of the effectiveness of these control campaigns for this endemic area.
The objective of the present study was to analyse the epidemiology of hydatid disease in western Spain from January 1998 to December 2012 by review of the diagnosed cases of hydatid disease admitted to a tertiary referral hospital situated in the western Spanish province of Salamanca.
METHODS
A retrospective descriptive study of patients diagnosed with CE in the Complejo Asistencial Universitario de Salamanca (CAUSA) between January 1998 and December 2012 was designed. CAUSA is a tertiary-care hospital of the Autonomous Community of Castilla and Leon that serves the province of Salamanca. It covers an area of 12 350 km2 encompassing 362 municipalities with a population of 350 564 individuals (45% in the capital) located in western Spain. The population counts per year at the municipality level were obtained from the National Institute of Statistics (INE; http://www.ine.es/).
The clinical data were obtained from the Unit of Clinical Documentation of CAUSA. Diagnosis and classification of CE were assessed according to the criteria proposed by the World Health Organization Informal Working Group on Echinococcosis for CE [Reference Brunetti, Kern and Vuitton16]. We included in the study all patients who were diagnosed, according to ICD-9 (code 122·0 to 122·9) criteria. Residents from other regions of Spain who underwent surgery were excluded from the study. Patients with post-operative recurrence and records with missing data, such as age, gender or city of residence, were also excluded from the study.
The clinical and epidemiological data were collected after revision of the medical records. Patients were stratified according to parameters such as age (0–14, 15–44, 45–69, 70–99 years) and primary and secondary diagnosis. Primary diagnosis was defined when CE was the cause of admission. Secondary diagnosis was defined when CE was any diagnosis different from the cause of admission.
CE is a notifiable disease in the Castilla and Leon region. In order to compare our data with those officially registered, we obtained additional data about the incidence of hydatid disease through the ‘Notifiable Disease System’ over the same period (1998–2012) from the epidemiological surveillance network of Castilla and Leon (Junta de Castilla y Leon; http://www.jcyl.es/).
Statistical analysis
The epidemiological analysis of the data included calculating the two measures of disease frequency: the incidence rate of hydatid disease was calculated by dividing the number of new cases of the disease by the average population at risk per time interval (person-years) multiplied by 100 000 and is expressed as ‘cases/105 person-years’. The cumulative incidence was estimated by dividing the number of new cases of CE by the population at risk at the beginning of the period. This value is a proportion ranging between 0 and 1 and is expressed as a percentage (%). The denominators were obtained from the population census of each municipality per year (INE; http://www.ine.es/).
The statistical analysis included: the univariate analysis, the results are expressed as percentages with the corresponding 95% confidence interval (CI) for a proportion as a measure of precision (inferential statistics) for categorical variables and as the mean and standard deviation (s.d.) for continuous variables. With regard to the bivariate and multivariate analysis, the χ 2 test was used to compare associations between categorical variables, such as clinical and demographic variables and surgical interventions. The outcome measure is expressed as the odds ratio (OR) together with its 95% CI. Continuous variables were compared using Student's t test or the Mann–Whitney test for two groups, depending on whether the given variable had a normal distribution. In order to analyse the temporal distribution of CE, a conventional linear regression analysis was performed. Results were considered statistically significant when P < 0·05. All data were analysed with R v. 3.0.0 (R Foundation for Statistical Computing; http://cran.r-project.org/) and visually displayed using the Lattice package [Reference Sarkar17]. Visualization and analysis of spatial data were performed using the ggplot2 package [Reference Wickham18].
RESULTS
Clinical data related to hydatid disease
Between January 1998 and December 2012, 586 patients with new CE-related diagnosis codes 122·0–122·9 were registered in CAUSA. Of these 586 patients, 71 came from other regions of Spain to undergo surgery and were consequently excluded from the study. In addition, 44 case records contained incomplete data, and these patients were also excluded. According to the inclusion criteria, a total of 471 patients were included in the study. Of the 471 cases diagnosed with CE, 263 (55·8%) were male (the male/female ratio was 1·26), and the average age was 62·3 ± 19·5 years. Although CE was diagnosed in 44·3% of elderly individuals (aged >70 years), seven (1·5%) patients were children (0–14 years), and 90 (19·1%) patients were aged between 15 and 44 years.
Hydatid cyst was the main cause of hospitalisation and the primary diagnosis in 203 (43·1%) cases. Meanwhile, CE was a secondary diagnosis in 268 (56·9%) cases. No differences were observed regarding the sex of the patients and the CE diagnosis between patients with primary and secondary diagnoses. However, most of the primary diagnoses were found in patients younger than 69 years (OR 2·04, 95% CI 1·60–2·61, P < 0·001), whereas the secondary diagnosis was most frequently found in elderly patients (>70 years), as shown in Table 1. These data suggest that diagnosis of CE in the elderly is usually related to other comorbidities.
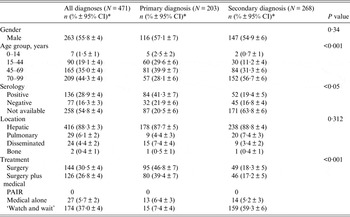
Table 1. Clinical and epidemiological features of 471 cases diagnosed with cystic echinococcosis grouped according to primary or secondary diagnosis
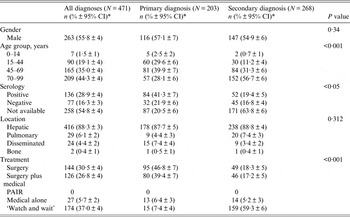
CI, Confidence interval; PAIR, percutaneous aspiration, injection and reaspiration.
* Percentage ±95% confidence interval for a proportion.
A single cystic lesion with a mean size of 8·0 cm (s.d. = 4·3) was presented in 447/471 (95·0%) patients, while the remaining 24/471 (5·0%) were diagnosed with a disseminated form of CE in which the average number of hydatid cysts was 2·4 (s.d. = 0·7). The hydatid serology test was performed for 213 patients, and we found that 136/213 (63·8%) patients presented positive (>1/80) anti-CE IgG titres, ranging from 1/80 to 1/10 240 (Table 1).
Surgery alone and surgery followed by treatment with anthelmintic drugs were the two main therapeutic approaches pursued in 46·8% and 39·4% of the CE primary diagnoses, respectively (Table 1).
Meanwhile, a ‘watch and wait’ option was chosen for 59·3% of secondary CE diagnoses (P < 0·001). These data show that the choice of surgery, and surgery followed by medical treatment in CE is related to the patients' age because the secondary CE diagnosis was clearly associated with an elderly population (>70 years) (P < 0·001). Furthermore, we observed that 138/354 patients were followed for a mean of 16·8 (s.d. = 7·1) months and that 47/276 (17·0%) of patients had recurrent hydatid disease.
Epidemiological CE data
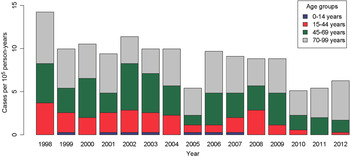
The incidence of CE in Salamanca during 1998–2012 was 8·9 cases/105 person-years (males 10·3 cases/105 person-years vs. females 7·7 cases/105 person-years) with a cumulative incidence of 0·24%. The highest incidence of CE occurred in 1998 with 14·2 cases/105 person-years, whereas the incidence decreased to 5·1 and 5·4 cases/105 person-years in 2010 and 2011, respectively, as shown in Figure 1. More importantly, a significant decrease in hydatid incidence was detected during the years included in the study (β = −0·4357, P < 0·001).
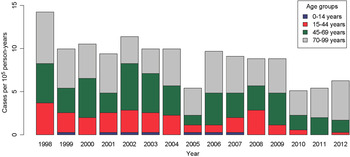
Fig. 1. Temporal trend of cystic echinococcosis incidence rates/100 000 individuals in Salamanca province.
On the other hand, a total of 175 cases were identified in the systematic search through the Notifiable Disease System during 1998–2012. Consequently, the mean incidence of hydatid disease in 1998–2012 obtained through the retrieval process was significantly higher than that provided by data from the Notifiable Disease System in Salamanca province (8·9 vs. 3·3 cases/105 person-years, P < 0·05). These data suggest that the incidence of hydatid disease in our region has been underestimated.
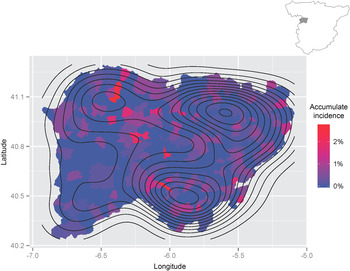
The geographical distribution of the cumulative incidence of hydatid disease in Salamanca province can be observed in Figure 2. We detected three geographical areas with a high cumulative incidence of hydatid disease, namely the capital (Salamanca) in the northeast and two other areas in the southern and northwestern areas of the province. In the capital, the cumulative incidence is significantly lower than in rural areas (P < 0·001). More importantly, it should be noted that the cumulative incidence of hydatid disease reached 2·76% in some rural areas. Most patients came from rural areas (310/471, 65·8%), whereas fewer cases (161/471, 34·2%) originated from urban areas. The cumulative incidence of hydatidosis in municipalities with <5000 inhabitants was higher than in municipalities of >5000 inhabitants (P < 0·05). Moreover, six municipalities with populations >5000 inhabitants accounted for 230/471 (48·83%) cases.
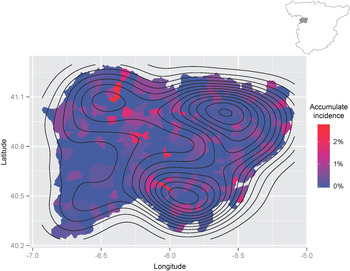
Fig. 2. Geographical distribution of hydatidosis in Salamanca province. The contours represent the density estimation of the prevalence of hydatid disease during 1998–2012.
DISCUSSION
Although CE is one of the most important existing anthropozoonosis in Spain, few data focused on the incidence and prevalence of this disease in humans are available [Reference Rojo-Vazquez13]. In the province of Salamanca, with an extensive or semi-extensive livestock farming industry, human CE remains highly endemic [Reference Pardo14]. In 2005, our group reported the first study focused on the incidence of human hydatidosis in the province of Salamanca [Reference Pardo14]. Suspecting that the incidence of human CE had not decreased, we updated the report in this study. The present work expands the analysis from 8 to 15 years and aims to provide new scientific evidence to generate policies that will help to analyse the impact of hydatid disease in our region.
Regarding the diagnosis of hydatid disease, we found that a primary CE diagnosis was frequently given to young patients, whereas a secondary accidental CE diagnosis was most frequently found in the elderly population and usually associated with other pathologies. Furthermore, the ‘watch and wait’ strategy and medical treatment alone were the treatment options most frequently pursued in the elderly population. These data suggest that the diagnosis of CE in elderly people is usually minimized. In spite of being traditionally considered as a ‘benign’ pathology, CE is an important cause of morbi-mortality [Reference Belhassen Garcia3]. Therefore, an expectant management of the disease can be dangerous, and it must only be employed for selected patients.
A systematic search of definitive CE cases obtained a disease incidence that was significantly higher than that acquired from data of the Notifiable Disease System; this result should provide motivation to follow the example set by the European Registry for Alveolar Echinococcosis and improve national registers for CE [Reference Kern19]. These records could be used as a tool to prioritize control measures for what is essentially a preventable disease.
In the present study, we found a high incidence of CE disease (8·9 cases/105 person-years) and a high cumulative incidence (2·76%) in some rural areas. These figures were significantly greater that those found in northern Spain [Reference Carabin20]. Our results could be initially attributed to the chronicity of the disease as well as the increased use of diagnostic imaging and serological methods. However, the presence of 20% of cases in patients aged <45 years and the persistence of paediatric cases suggest that a high rate of infection is being sustained in the population. In our study, we also detected similar disease incidences in both sexes, suggesting that the occupational component of the risk is less relevant than other environmental risk factors [Reference Campos-Bueno, López-Abente and Andrés-Cercadillo21]. Furthermore, a significant decrease in hydatid incidence was detected during the time period included in the study. The highly endemic nature of hydatidosis in the province of Salamanca is a consequence of the E. granulosus cycle persistence over many years. Health education campaigns based on changing risk behaviours, such as elimination of stray dogs, reduction of parasite burden in the definitive hosts by praziquantel administration and the removal of animal corpses, are the principal measures for the prevention of CE infection.
Most patients with CE live in rural areas with a wide geographical distribution. This geographical heterogeneity of CE infection has also been reported in numerous countries; therefore, it is difficult to identify the relevant risk factors for this disease in our province, region and country [Reference Acosta-Jamett22, Reference Ito23]. Despite the wide distribution of cases in our region, we found a higher cumulative incidence in rural areas than in urban areas, and this pattern of CE infection has also been documented in previous studies [Reference Campos-Bueno, López-Abente and Andrés-Cercadillo21].
The main limitation of our work was the initial selection bias. The present study only considers the cases admitted to CAUSA; cases diagnosed in private clinics and primary-care practices were not included in the study. Therefore, we can assume that the actual incidence of human hydatidosis in the province of Salamanca is even higher than that estimated in the present study. Furthermore, our study shows that the percentage of surgical cases in our hospital is <60% of the total of CE diagnoses. Therefore, these data suggest that the studies based on surgical cases underestimate the true incidence of human hydatidosis.
It can be concluded that CE incidence in Salamanca province has diminished in recent years, although active transmission remains in paediatric and young patients, and the diagnosis of CE in the elderly population is usually minimized. CE incidence remains high in our region despite public health plans for disease control. Furthermore, the Notifiable Disease System showed an incidence of CE disease that clearly underestimated the real numbers. These data suggest the need for increased monitoring and control of CE.
ACKNOWLEDGEMENTS
The authors acknowledge the Unit of Clinical Documentation of CAUSA for their help with data collection.
This work should be attributed to Centro de Investigación de Enfermedades Tropicales de la Universidad de Salamanca (CIETUS), IBSAL, Salamanca, Spain.
DECLARATION OF INTEREST
None.