Gestational diabetes mellitus (GDM), carbohydrate intolerance and insulin resistance during pregnancy are serious problems that are increasing worldwide, which carry significant short-term and long-term adverse health outcomes in both mother and offspring( Reference Zhang, Rawal and Chong 1 ). In GDM women, the physiological changes in insulin resistance and lipid profiles are exaggerated and may indicate an underlying metabolic dysfunction that transiently manifests during pregnancy( Reference Carpenter 2 ). In addition, a few studies have postulated that changes in the human gut microbiome are associated with metabolic diseases including type 2 diabetes mellitus (T2DM), insulin resistance( Reference Greenway, Wang and Heiman 3 ) and increased inflammatory cytokines( Reference Koren, Goodrich and Cullender 4 ).
The potential role of the gut flora as well as synbiotics in decreased insulin resistance and decreased levels of cholesterol and TAG resulted in an interest in using synbiotics as preventive and therapeutic interventions. Probiotics are live bacteria and yeasts that confer health benefits to the host when consumed in sufficient quantities( Reference Fuller 5 ). Furthermore, prebiotics such as inulin and fructo-oligosaccharides (FOS) that stimulate the growth and metabolism of probiotics in the gut have also been found to a exhibit favourable role against several gut-related complications( Reference Gibson and Roberfroid 6 ). The beneficial effects of synbiotics on markers of insulin metabolism and lipid concentrations in patients with T2DM( Reference Shakeri, Hadaegh and Abedi 7 ) and in subjects with the metabolic syndrome( Reference Eslamparast, Zamani and Hekmatdoost 8 ) have been previously reported. We have shown that consumption of a synbiotic food containing Lactobacillus sporogenes (18×107 colony-forming units (CFU)) plus 0·72 g inulin for 9 weeks by pregnant women had beneficial effects on markers of insulin metabolism, but did not affect fasting plasma glucose (FPG) concentrations( Reference Taghizadeh and Asemi 9 ). In addition, a significant reduction in total cholesterol, LDL-cholesterol and TAG and an increase in HDL-cholesterol were observed following the consumption of synbiotics containing Lactobacillus salivarius with FOS among healthy, young individuals for 6 weeks, but it did not influence insulin resistance( Reference Rajkumar, Kumar and Das 10 ).
Intake of synbiotics may improve markers of insulin resistance and lipid profiles in GDM patients through the production of SCFA, carbon disulfide and methyl acetate( Reference Vitali, Ndagijimana and Maccaferri 11 ) and decreased expression of inflammation-related genes( Reference Voltolini, Battersby and Etherington 12 ). To our knowledge, data on the effects of synbiotic supplementation on markers of insulin metabolism and lipid concentrations in patients with GDM are scarce. The objective of this study was to evaluate the effects of synbiotic supplementation on markers of insulin metabolism and lipid profiles in these patients.
Methods
Trial design
The present study was a 6-week prospective, randomised, double-blind, placebo-controlled clinical trial.
Participants
In the present study, we included seventy women with GDM aged 18–40 years without previous diabetes, who were diagnosed with GDM by ‘one-step’, 2-h, 75-g oral glucose tolerance test (OGTT) at 24–28 weeks of gestation, referred to Kosar Clinic in Arak, Iran, from March 2016 to May 2016. We diagnosed GDM on the basis of the American Diabetes Association guidelines( 13 ). Patients who met one of the following criteria were considered as having GDM: FPG≥92 mg/dl, 1-h OGTT≥180 mg/dl and 2-h OGTT≥153 mg/dl( 13 ). Exclusion criteria were as follows: subjects taking synbiotic or probiotic supplements, including probiotic yogurt, kefir and other fermented foods, subjects taking insulin, subjects with placenta abruption, pre-eclampsia, eclampsia, hypothyroidism and hyperthyroidism, smokers, and those with kidney or liver diseases.
Ethics statements
This trial was performed according to the principles of the Declaration of Helsinki, and the study protocol was approved by the ethics committee of AUMS (reference number IR.ARAKMU.REC.1395.22). The study protocol was carefully explained to all subjects before obtaining informed consent. The current study is registered in the Iranian website (www.irct.ir) for registration of clinical trials (http://www.irct.ir: IRCT201605085623N77).
Study design
At the onset of the study, patients were first matched one by one according to age and BMI. Next, the matched patients were randomly assigned to the intervention and placebo groups. The participants were randomly allocated into two treatment groups to take either synbiotic supplements (n 35) or placebo (n 35) for 6 weeks. At the onset of the study, patients were requested not to change their routine physical activity or usual dietary patterns throughout the study and not to consume any supplements other than the ones provided to them by the investigators, as well as not to take any medications that might affect findings during the 6-week intervention. Dietary macronutrient and micronutrient intakes were assessed using 3-d food records (comprised of 2 working days and a weekend day) at study onset, weeks 3 and 5, and at the end of the intervention. All participants completed the physical activity records at weeks 0, 3 and 6 of the intervention. Modified Nutritionist-4 software programme (First Databank) was used to estimate the energy and nutrient intakes. In the present study, physical activity was described as metabolic equivalents (MET) in h/d. To determine the MET for each patient, we multiplied the times (h/d) reported for each physical activity by the related MET coefficient using standard tables( Reference Ainsworth, Haskell and Whitt 14 ). Questionnaires were used to measure physical activity levels.
Intervention
The synbiotic capsules contained Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum (2×109 CFU/g each) plus 0·8 g inulin. Participants in the placebo group received capsules containing starch without bacteria and inulin. It is well known that it would be more appropriate if the strains used in probiotic supplements for human consumption are derived from the human intestinal tract, are well characterised, are able to outlive the rigours of the digestive tract and possibly colonise, are biologically active against the target, and are stable and amenable to commercial production and distribution( Reference Soccol, Vandenberghe and Spier 15 ). Owing to the lack of evidence about the appropriate dosage of probiotics and inulin for GDM women, we used the above-mentioned doses of probiotics based on a few previous studies in healthy subjects( Reference Mohammadi, Jazayeri and Khosravi-Darani 16 , Reference Benton, Williams and Brown 17 ), and the dose of inulin was based on a previous study in healthy pregnant women( Reference Taghizadeh and Asemi 9 ). The appearance of the placebo was indistinguishable with regard to colour, shape, size and packaging, smell and taste from the probiotic capsule. All capsules were produced by Tak Gen Zist Pharmaceutical Company, which is approved by Food and Drug Administration.
Treatment adherence
Every 2 weeks, individuals were provided sufficient synbiotic and placebo capsules to last 3 d after their next scheduled visit and were instructed to return all unused supplements at each visit. The remaining supplements were counted and subtracted from the number provided to determine the number taken. To increase compliance, all participants received short messages on their cell phones as a reminder to take the supplements every day.
Assessment of anthropometric measurements
Weight and height of participants were determined in an overnight fasting status using a standard scale (Seca) at the onset of the study and after 6 weeks’ intervention. BMI was calculated using the height and weight measurements (weight in kg/(height in metres)2).
Assessment of outcomes
In the present study, the primary outcome measurements were markers of insulin metabolism, and the secondary outcome measurements were lipid concentrations.
Biochemical assessment
Blood samples were collected at weeks 0 and 6 after at least 12 h of fasting. All blood samples were centrifuged at 3000 g for 10 min, and serum samples were separated into clean tubes and stored at −80°C until analysis at the AUMS reference laboratory. To determine FPG, serum TAG, VLDL-cholesterol, total-cholesterol, LDL-cholesterol and HDL-cholesterol concentrations, we used enzymatic kits (Pars Azmun). All inter- and intra-assay coefficients of variances (CV) for FPG and lipid concentrations were <5 %. Circulating levels of serum insulin were determined using ELISA kits (Monobind) with intra- and inter-assay CV of 3·0 and 4·7 %, respectively. The homoeostatic model assessment for insulin resistance (HOMA-IR), homoeostatic model assessment for B-cell function (HOMA-B) and the quantitative insulin sensitivity check index (QUICKI) were determined according to suggested formulae( Reference Pisprasert, Ingram and Lopez-Davila 18 ).
Sample size
To calculate sample size, we used the standard formula suggested for parallel clinical trials after considering type I error (α) of 0·05 and type II error (β) of 0·20 (power=80 %). On the basis of a previous study( Reference Taghizadeh and Asemi 9 ), we used 1·8 as sd and 1·3 as the difference in mean (d) of HOMA-IR as key variables. Therefore, we needed thirty persons in each group. Assuming five dropouts in each group, the final sample size was determined to be thirty-five subjects per group.
Randomisation
Randomisation was carried out using computer-generated random numbers. Randomisation and allocation were concealed from the researchers and participants until all the analyses were completed. The randomised allocation sequence, enrolling participants and allocating them to interventions were conducted by a trained staff at the gynaecology clinic.
Statistical methods
Normality variables were evaluated by the Kolmogorov–Smirnov test. All values are expressed as means and standard deviations. The data were analysed according to the intention-to-treat (ITT) principle using the Statistical Package for Social Science, version 18 (SPSS Inc.). Differences in general characteristics and daily macronutrient and micronutrient intakes between the two groups were tested by independent sample t test before and after intervention. Differences within a group before and after intervention were analysed by paired sample t test. To determine the effects of synbiotic intake on markers of insulin resistance and lipid concentrations, one-way, repeated-measures ANOVA was used to evaluate the between-group changes in variables during the study. To assess whether the magnitude of the change depended on the baseline values of biochemical parameters, maternal age and baseline BMI, we adjusted all analyses for these variables to avoid the potential bias that might have resulted. These analyses were carried out using ANCOVA. P values<0·05 were considered to be statistically significant.
Results
At baseline, we recruited ninety patients; however, twenty subjects were excluded from the study as they did not meet inclusion criteria. As demonstrated in the study flow diagram (Fig. 1), during the intervention phase of the study, three participants were excluded from the placebo group (withdrawn because of personal reasons (n 3)). In addition, one subject was excluded from the synbiotic group (withdrawn because of personal reasons (n 1)). However, as the analysis was performed on the basis of the ITT principle, all seventy subjects with GDM were included in the final analysis. On average, the rate of compliance in our study was high, as more than 90 % of the probiotic capsules were consumed throughout the study in both groups. No side-effects were reported following supplementation with synbiotics in GDM patients throughout the study.

Fig. 1 Summary of patient flow.
Mean age, height, baseline weight and BMI as well as their means after 6 weeks of intervention were not significant between the synbiotic group and the placebo group (Table 1).
Table 1 General characteristics of the study participants (Mean values and standard deviations)
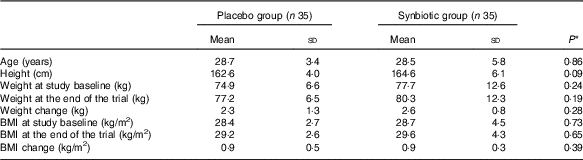
* Obtained from independent t test.
Comparison of dietary 3-d intakes of study participants throughout the study revealed no significant differences in macronutrient and micronutrient intakes including energy, carbohydrates, proteins, fats, SFA, PUFA, MUFA, cholesterol, total dietary fibre, Fe, Mg, Zn and Mn between the two groups (Table 2).
Table 2 Dietary intakes of study participants throughout the study (Mean values and standard deviations)
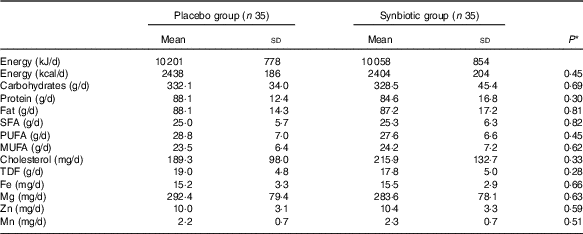
TDF, total dietary fibre.
* Obtained from independent t test.
After 6 weeks of intervention, compared with the placebo, synbiotic supplementation led to a significant decrease in serum insulin levels (−1·5 (sd 5·9) v. +4·8 (sd 11·5) µIU/ml, P=0·005), HOMA-IR (−0·4 (sd 1·3) v. +1·1 (sd 2·7), P=0·003) and HOMA-B (−5·1 (sd 24·2) v. +18·9 (sd 45·6), P=0·008) and a significant increase in QUICKI (+0·01 (sd 0·01) v. −0·007 (sd 0·02), P=0·02) (Table 3). In addition, synbiotic intake significantly decreased serum TAG (−14·8 (sd 56·5) v. +30·4 (sd 37·8) mg/dl, P<0·001) and VLDL-cholesterol concentrations (−3·0 (sd 11·3) v. +6·1 (sd 7·6) mg/dl, P<0·001) compared with the placebo. We did not observe any significant change after synbiotic supplementation on FPG and other lipid profiles.
Table 3 Markers of insulin metabolism and lipid profiles at study baseline and after 6 weeks of intervention in patients with gestational diabetes mellitus who received either synbiotic supplements or placebo (Mean values and standard deviations)

FPG, fasting plasma glucose; HOMA-IR, homoeostasis model assessment for estimated insulin resistance; HOMA-B, homoeostasis model assessment for estimated B cell function; QUICKI, quantitative insulin sensitivity check index.
* Obtained from paired-sample t tests.
† Obtained from repeated-measures ANOVA test.
Baseline concentrations of total cholesterol differed significantly between the two groups. Therefore, baseline concentrations of biochemical variables, maternal age and BMI at baseline were controlled for the analyses. However, after this adjustment, no significant changes in our findings were observed (Table 4).
Table 4 Adjusted changes in metabolic variables in patients with gestational diabetes mellitus who received either synbiotic supplements or placebo (Mean values with their standard errors)
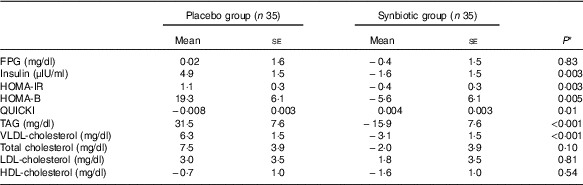
FPG, fasting plasma glucose; HOMA-IR, homoeostasis model assessment for estimated insulin resistance; HOMA-B, homoeostasis model assessment for estimated B cell function; QUICKI, quantitative insulin sensitivity check index.
* Obtained from ANCOVA test adjustment for baseline values plus maternal age and baseline BMI.
Discussion
In the current study, we investigated the effects of synbiotic supplementation on markers of insulin metabolism and lipid profiles in patients with GDM. We found that the intake of probiotic supplements for 6 weeks among patients with GDM had beneficial effects on markers of insulin metabolism, TAG and VLDL-cholesterol concentrations; however, it did not influence FPG and other lipid profiles.
GDM women are susceptible to insulin resistance, dyslipidaemia and increased risk of adverse short- and long-term health outcomes( Reference Asemi, Samimi and Tabassi 19 ). This is therefore a good group to target with lifestyle interventions including synbiotic supplementation during pregnancy. Our study showed that synbiotic supplementation for 6 weeks in GDM women resulted in a significant reduction in serum insulin concentrations, HOMA-IR and HOMA-B and a significant increase in QUICKI compared with the placebo, but FPG remained unchanged. Although the beneficial effects of synbiotic intake on markers of insulin resistance on patients without GDM and animal models have been evaluated, to the best of our knowledge, this study is the first to assess the favourable effects of synbiotic supplementation on markers of insulin resistance in patients with GDM. In agreement with our findings, synbiotic supplementation containing 200 million of seven strains of friendly bacteria plus FOS for 28 weeks in subjects with the metabolic syndrome led to significant improvements in insulin resistance parameters( Reference Eslamparast, Zamani and Hekmatdoost 8 ). In addition, Malaguarnera et al.( Reference Malaguarnera, Vacante and Antic 20 ) noticed a significant reduction in the HOMA-IR following intake of Bifidobacterium longum plus FOS for 24 weeks among patients with non-alcoholic steatohepatitis, but no significant changes in serum insulin levels were observed. In another study, Yadav et al.( Reference Yadav, Jain and Sinha 21 ) observed that probiotic dahi-supplemented diet significantly delayed the onset of glucose intolerance, hyperglycaemia and hyperinsulinaemia in high fructose-fed rats. However, a few researchers were unable to observe such beneficial effects of probiotic supplementation on glycaemic status. For instance, a probiotic capsule intervention containing 109 CFU of L. salivarius among GDM women for 4–6 weeks had no impact on glycaemic control( Reference Lindsay, Brennan and Kennelly 22 ). Hyperinsulinaemia and hyperglycaemia in GDM can result in progression to T2DM later in life and neonatal complications( Reference Greenberg 23 , Reference Asemi, Karamali and Esmaillzadeh 24 ). Synbiotic intake might improve glucose homoeostasis parameters through modification of gut flora, reduction of endotoxin levels, reduction of production and absorption of intestinal toxins, elevation of faecal pH( Reference Compare, Coccoli and Rocco 25 ) and reduction of pro-inflammatory cytokine production( Reference Li, Yang and Lin 26 ).
This study demonstrated that taking synbiotic supplements for 6 weeks by GDM women was associated with a significant reduction in serum TAG and VLDL-cholesterol concentrations, but did not influence other lipid profiles. We have previously shown that β-carotene fortified synbiotic food intake containing L. sporogenes (27×107 CFU) and 2·7 g inulin in patients with T2DM for 6 weeks decreased serum TAG and VLDL-cholesterol concentrations; however, it did not affect other lipid profiles( Reference Asemi, Alizadeh and Ahmad 27 ). Furthermore, synbiotic supplementation containing L. casei, Lactobacillus r.hamnosus, Streptococcus thermophilus, Bifidobacterium breve, L. acidophilus, Bifidobacterium longum and Lactobacillus bulgaricus (2·0×108 CFU each) plus FOS among obese children for 8 weeks resulted in a significant decrease in serum TAG and total- and LDL-cholesterol levels( Reference Safavi, Farajian and Kelishadi 28 ). In another study, Schaafsma et al.( Reference Schaafsma, Meuling and van Dokkum 29 ) observed that a combination of probiotic L. acidophilus plus FOS supplementation significantly reduced total-cholesterol, LDL-cholesterol and LDL-cholesterol/HDL-cholesterol levels, while it did not influence TAG levels. In a similar study on men and women with hypercholesterolaemia, a significant reduction in total- and LDL-cholesterol concentrations was seen after 12 weeks of supplementation with a combination of L. acidophilus plus prebiotic inulin( Reference Ooi, Ahmad and Yuen 30 ). Both increased maternal TAG and free fatty acid levels in GDM women correlated with fetal growth during pregnancy and with neonatal anthropometric measures( Reference Schaefer-Graf, Graf and Kulbacka 31 ). In addition, previous studies have reported that maternal lipid profile alterations are associated with complications such as macrossomia( Reference Kitajima, Oka and Yasuhi 32 ), pre-eclampsia( Reference Cekmen, Erbagci and Balat 33 ) and preterm birth( Reference Vrijkotte, Krukziener and Hutten 34 ). Different study designs, characteristics of study persons, different dosages, types and quality of the used probiotic bacteria and inulin as well as duration of the intervention are some reasons for discrepant findings. Synbiotic intake may decrease TAG and VLDL-cholesterol concentrations by lipolysis of TAG and transform TAG-rich particles into small particles( Reference Matsuoka, Miura and Hori 35 ), suppressing the NF-κ light-chain-enhancer of activated B cells pathway( Reference Shi, Kokoeva and Inouye 36 ) and gut microbiota–SCFA–hormone axis( Reference Yadav, Lee and Lloyd 37 ). In addition, probiotic and inulin intake may improve insulin resistance and lipid profiles through up-regulation of the PPAR-γ gene( Reference Deepak, Ram Kumar Pandian and Sivasubramaniam 38 , Reference Dewulf, Cani and Neyrinck 39 ). In their study, Wang et al.( Reference Wang, Xie and Li 40 ) observed that L. casei treatment significantly increased the expression of PPAR-γ gene in a rat model of acute liver failure induced by lipopolysaccharide and d-galactosamine for 30 d. PPAR-γ is a member of the nuclear receptor superfamily and regulates the expressions of several genes encoding proteins involved in adipocyte differentiation, fatty acid storage and glucose metabolism( Reference Grindflek, Sundvold and Lien 41 ). Down-regulation in the expressions of hepatic genes involved in lipogenesis and fatty acid elongation/desaturation by inulin may also result in improvement in lipid profiles( Reference Weitkunat, Schumann and Petzke 42 ).
Limitations of the current study include the absence of faecal sample data to demonstrate transit of the specific probiotic through the gastrointestinal tracts of study subjects in the intervention group. In addition, we did not use the faecal samples for colony count of specific probiotics. Because of funding limitations, we did not evaluate the expressed levels of related variables with insulin resistance and lipid profiles.
Overall, the results of this study demonstrate that taking synbiotic supplements for 6 weeks by patients with GDM had beneficial effects on markers of insulin metabolism, TAG and VLDL-cholesterol concentrations; however, it did not affect FPG and other lipid profiles. This suggests that synbiotic supplementation may confer advantageous therapeutic potential for patients with GDM. Further research is needed in other patients and for longer periods to determine the safety of this supplemental approach. In addition, further studies are needed to evaluate the expressed levels of related variables with insulin resistance and lipid profiles to explore the plausible mechanism and confirm our findings.
Acknowledgements
The present study was funded by a grant from the Vice-chancellor for Research, AUMS, and Iran. The authors would like to thank the staff of Kosar Clinic (Arak, Iran) for their assistance on this project.
Z. A. contributed to the conception, design, statistical analysis and drafting of the manuscript. S. A., M. J., M. T.-E. and P. J. contributed to the conception, data collection and manuscript drafting. The final version was confirmed by all authors before submission.
The authors declare that there are no conflicts of interest.








