Maximum oxygen consumption displays a wide variability in adult patients with CHD and is an independent prognostic factor. The single most important determinant of maximum oxygen consumption is the type of anatomy. Reference Diller, Dimopoulos and Okonko1 The expected trend of functional capacity in patients with CHD is to decline over time leading to significantly reduced maximum oxygen consumption as compared with the age-matched general population, irrespective of NYHA class. Such decline may have several causes, namely chronotropic incompetence, absence of subpulmonary ventricle, muscular deconditioning, balance between systemic, and pulmonary resistances, depending on anatomy.
Exercise training, by improving muscular metabolism, optimising body mass index, and promoting systemic and pulmonary vasodilatation, is an attractive therapeutical intervention in patient with CHD. Reference Rhodes, Ubeda Tikkanen and Jenkins2 Nevertheless, evidence from the literature is still inconclusive showing improvement of functional capacity limited to only specific anatomies. Reference Duppen, Takken and Hopman3
Furthermore, different from adult CHD, reference value of maximum oxygen consumption based on large datasets has not been established so far in adolescent and young adults. Reference Paridon, Mitchell and Colan4–Reference Engelfriet, Boersma and Oechslin6
This study aims to investigate cardiopulmonary exercise parameters in a cohort of young adult with CHD involved in physical training programme and to describe their long-term trend. The second objective of the study is to compare exercise metabolic parameters of individuals with CHD undergoing a regular physical training programme with a matched control group.
Methods
Patients with CHD and involved in sports activities either at recreational or competitive level were evaluated at the sports medicine department between 2011 and 2019. Competitive and recreational (or not competitive) levels were defined according to the criteria established by the Italian law. Patients belonging to a specific federation and undergoing training sessions and competitions on a defined schedule were considered as “competitive” while those performing physical activities on regular basis (at least twice a week) but not participating in formal events were labelled as “recreational.” They all underwent serial cardiopulmonary exercise tests.
Ergometric tests were performed on a cycloergometer. Exercise protocol was tailored on the age and patient’s weight. Patients exercised following a ramp protocol, with the workload selected according to the maximum theory.
Continuous 12 leads ECG and transcutaneous saturation were monitored during exercise while blood pressure was recorded every 2 minutes.
Ventilatory parameters were measured by a turbine coupled with a digital trasducer. Expired gas analysis was performed with a Vyntus CPX metabograph CareFusion™ using Rudolph masks adjusted to the face size.
All ventilation and metabolic parameters were recorded beat by beat and analysed averaging ten seconds.
The last cardiopulmonary exercise test was compared with the oldest one, conducted with the same protocol, whenever available, in order to assess exercise capacity trend. The following parameters were included: functional capacity expressed as maximum workload in Watts, maximum heart rate; maximum oxygen consumption (max VO2 ml/kg/min); oxygen pulse (VO2/HR), CO2 ventilatory equivalent (VE/VCO2) and VO2/Work slope. Ventilatory equivalent of CO2 slope was calculated between the 20 % and 80% of the entire exercise in order to exclude possible spurious estimations due to hyperventilation at the beginning of the exercise. All tests were conducted aiming at a respiratory exchange ratio higher than 1.1, that was assumed as index of adequate metabolic stress. Individuals practising sports were matched 1:1 for anatomical diagnosis, age (±2 years) and body mass index (±2) with a control cohort. Patients with haemodynamically significant residual defects, cardiovascular symptoms at rest, NYHA class > 2 were excluded in order to avoid confounders.
This study was approved by institutional review board (24/INT/2023).
Statistical analysis
Quantitative variables are expressed as mean ± SD or median (interquartile range) and compared with Student’s t-test or Wilcoxon rank sum test. Comparison of quantitative observations at two different timeframes was performed by Student’s t-test or Wilcoxon test for paired data. Normality of variables was assessed by visually inspecting the distribution histograms.
Categorical variables are expressed as counts or percentage and compared by chi-square or Fisher’s exact test as appropriate. A p value < 0.005 was assumed as cut-off for statistical significance.
Statistical analysis was performed by STATA software.
Results
Among one hundred and eleven patients, 73 males (66%) were included in the analysis. Median age at last cardiopulmonary exercise test was 14 (12–17) years. Median body mass index of the whole cohort was 19.7 (17–21). Twenty-nine patients (27%) have been practising sports at competitive level for a median time of 4 (2–7) years; for two patients, the information about the level of training involvement was not available. In the whole population, individuals performing sports at competitive level had a similar age and body mass index which displayed a lower prevalence of great complexity defects as compared to the “recreational training” group (Table2 suppl). Underlying anatomic diagnosis were transposition great arteries post-arterial switch operation (21 patients, 19%); tetralogy of Fallot (15 patients, 13%); aortic coarctation/ left heart obstructions (31 patients, 28%); atrioventricular septal defect (7 patients, 6%); Fontan (5 patients, 4%); pulmonary stenosis (9 patients, 8%); other diagnosis (18 patients, 16%). Thirteen patients (13%) were untreated while 31 patients (32%) had residual defects. (Table 1).
Table 1. Demographic data. IQR: interquartile range, BMI: body mass index, TGA-ASO: transposition great arteries-arterial switch operation, TOF: tetralogy of Fallot, CoA: aortic coarctation, AVSD: atrioventricular septal defect.

Table 2 summarises demographic and cardiopulmonary performance parameters according to the different anatomic categories. Maximum oxygen consumption and % predicted oxygen consumption displayed a significant variability among different underlying anatomies. (Fig. 1).

Figure 1. Maximum oxygen consumption (panel A) and percentage of maximum predicted oxygen consumption (panel B) according to anatomic diagnosis. TGA-ASO: transposition great arteries-arterial switch operation, AVSD = atrioventricular septal defect; CoA = aortic coarctation; TOF = tetralogy of Fallot.
Table 2. Demographic, metabolic, and respiratory parameters according to categories of diagnosis. W: IQR: interquartile range, workload, VO2: oxygen consumption, VE/VCO2: ventilatory equivalent of CO2.

In fifty patients, more than one cardiopulmonary exercise test was available. In this subgroup, the last test was compared with the oldest one. Median time elapsed between the two tests was 1007 (663–1471) days.
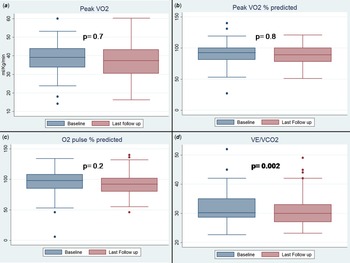
Absolute value of maximum oxygen consumption and its predicted percentage at last test were not significantly different from the antecedent one: maximum oxygen consumption 38.2 ± 9 versus 38.6 ± 9.2 ml/Kg/min (p = 0.7); last % oxygen consumption 88 ± 20 versus 87 ± 15 (p = 0,8), oxygen consumption/Work 10 ± 2.4 versus 10 ± 1.6 (p = 0.9).
On the other hand, ventilatory equivalent of CO2 significantly decreases in the last test as compared with the baseline: 30 ± 4 versus 33 ± 5 (p = 0.002). (Table 3) (Fig. 2).

Figure 2. Pairwise comparison of maximum oxygen consumption (panel A) and percentage of maximum predicted oxygen consumption (panel B), percentage of maximum predicted O2 pulse (panel C) and ventilatory equivalent of CO2 (panel D).
Table 3. VO2: oxygen consumption, VE/VCO2: ventilatory equivalent of CO2, AT: anaerobic threshold, HR: heart rate, FEV1: first-second forced expiratory volume.

After matching, 46 couples sports practising/non-sports practising were analysed. As a result of the matching, age and body mass index were not significantly different between the two groups. Patients practising sports achieved a higher degree of metabolic stress as showed by a significantly higher median maximum respiratory exchange ratio: 1.17 (1.07–1.22) versus 1.10 (1.05–1.14), p = 0.04. Maximal workload, oxygen consumption and percentage of predicted maximum oxygen consumption were significantly higher in patient involved in sports activities: 141 (120–160) versus 110 (90–130) W, 36.75 (30.2–42.1) versus 28.7 (24–34.7) ml/Kg/min p < 0.001 and 84.5 (75–97) versus 61.5 (52–72)%, respectively. All p < 0.001.
On the other hand ventilatory equivalent of CO2 was not significantly different 29.2 (26.6–32) versus 27.7 (25–32.9) p = 0.4 (Table 4).
Table 4. Comparison between patients with CHD undergoing physical training and control group.

No complication related to the test occurred.
Discussion
Assessment of functional capacity has a well-consolidated role in the prognostic stratification of CHD patients. In our cohort of trained patients with CHD, maximum oxygen consumption was well preserved across all categories of anatomies, ranging from 39 (36–44) to 28 (23–32) ml/Kg/min. In a previous large dataset of adult patients with CHD, maximum oxygen consumption ranged from 28.7 ± 10.4 to 11.5 ± 3.6 ml/Kg/min. Reference Diller, Dimopoulos and Okonko1 This significant difference may be explained by a higher level of training, a lower age of our population, and the exclusion of patients with Eisenmenger physiology. However, previous study on a group of children and adolescent with CHD also reported a maximum workload significantly lower than our cohort. Reference Norozi, Gravenhorst, Hobbiebrunken and Wessel5
A main finding of this paper is that patient practising sport, despite a similar reported NYHA class, displayed a significantly higher objective functional capacity as compared with their counterpart with the same age, anatomy, and body mass index. Interestingly, ventilatory equivalent of CO2 was not significantly different in subjects practising sport. This finding can be the result of the specific characteristics of our cohort which included young patients without pulmonary vascular disease.
Literature data on the effect of exercise training on cardiopulmonary fitness are still conflictual.
It has to be underlined that most of the studies assessing the impact of physical training on functional capacity of patients with CHD are based on heterogenous protocol of limited duration. Reference Duppen, Takken and Hopman3,Reference Amiard, Jullien, Nassif, Bach, Maingourd and Ahmaidi7–Reference Dirks, Kramer and Schleiger12
Different from previous studies, we included patients that were involved in sports activities as part of their routine lifestyle. We can hypothesise that a training protocol of limited duration might not reproduce the effect of routine training on effort tolerance at long-term follow-up. We observed that functional capacity in terms of maximum oxygen consumption and % predicted maximum oxygen consumption remained stable over the follow-up period while ventilatory equivalent of CO2 slope decreased. It has been demonstrated that a lower level of ventilatory equivalent of CO2 is independently associated with a better prognosis in patients with CHD. Reference Inuzuka, Diller and Borgia13 Efficiency of ventilation-perfusion coupling is a major component of ventilatory equivalent of CO2 slope. In patients with CHD, pulmonary vascular function may be impaired because of global segmental pulmonary hypertension, pulmonary artery branch stenosis, lack of flow pulsatility, contributing to regional ventilation mismatch, and, therefore, impairing ventilation efficiency. This is particularly relevant for specific categories of patients, such as Fontan, that have a passive not pulsatile pulmonary flow. Reference Banks, McCrindle, Russell and Longmuir14 Routine physical training might counteract these pathophysiological mechanisms by optimising NO-mediated pulmonary vascular recruitment. Reference Taylor, Coffman, Summerfield, Issa, Kasak and Johnson15
Finally, involvement in regular training and surveillance of objective effort tolerance by cardiopulmonary exercise test may increase the detection rate of subclinical but potentially harmful residual defects. (supplementary material).
Larger numbers are needed in order to confirm these trends of exercise parameters in sportive patients with CHD in order to be able to perform sub-analysis in different anatomic categories.
Conclusions
In summary, these data indicate that cardiopulmonary exercise test is an useful and safe tool to monitor patients regularly involved in physical training. This group displayed a better-preserved effort tolerance as compared with age- and anatomy-matched controls. Furthermore, physical exercise in patients with CHD may contribute to the preservation of functional capacity over the years. Positive effect of exercise on pulmonary circulation, peripheral oxygen extraction, and weight control can explain this finding.
Limitations
This is an observational retrospective study. Although we introduced a matched control group, our population is very heterogeneous in terms of underlying anatomic diagnosis; hence, the results might be biased by not considering confounders. Moreover, the sample size is too small and likely underpowered to clearly exclude any significant variation in metabolic parameters by a robust repeated measures variance analysis.
Acknowledgements
This work was supported by IRCCS Policlinico San Donato, a Clinical Research Hospital partially funded by the Italian Ministry of Health.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1047951123003621.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests
None.