Mineral nutrition
Mineral malnutrition is a global health concern and deficiencies in iron and zinc are particularly prevalent. Iron deficiency is present in one-third of the world's population(Reference Zimmermann and Hurrell1) and accounts for approximately 50 % of global cases of anaemia(2). The global incidence of zinc deficiency is estimated to be 15–20 %(Reference Wessells and Brown3) and is associated with several disorders including poor immune function and growth. Stunting associated with zinc deficiency affects more than 150 million children under age 5(4). Both iron and zinc are therefore regarded as nutrients of concern by The World Health Organisation(2,4) . While iron and zinc deficiencies are more prevalent in low- and middle-income countries, the rates of deficiency are also high in the UK. Iron deficiency (serum ferritin levels below 15 μg/l) affects up to 25 % of adolescent and pre-menopausal adult females(5), while up to 20 % of females in the UK may have mild-to-moderate zinc deficiency based on the International Zinc Nutrition Consultative Group thresholds for plasma zinc(6,7) .
According to the National Diet and Nutrition Survey(5), cereal products are major contributors to mineral intake in the UK. For example, in the diet of UK adolescent population groups, cereal products provide over 50 % of iron and more than 30 % of zinc(5). This includes the endogenous minerals present in foods and those added through fortification (e.g. of white starch and breakfast cereals). However, while cereal products may contribute significantly to dietary intake, the minerals contained in cereal products are poorly absorbed.
Mineral bioaccessibility
In this review, we have defined bioaccessibility and the amount of a nutrient released from food during digestion. Bioavailability (discussed later) is the amount of the released nutrient absorbed in the intestine and either stored within the body or utilised for metabolic purposes.
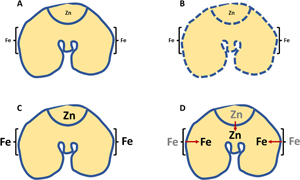
Mineral bioaccessibility from cereals is influenced by several factors including the localisation in plant tissues, the speciation (e.g. the chemical forms in which the mineral is stored), the composition of the plant cell walls and the type and degree of processing during the production of cereal products. Wheat is the major cereal grown and consumed in the UK. The structure of the wheat grain (or caryopsis) is highly specialised and the minerals in wheat are stored in specific tissues of the caryopsis. Iron is largely found in the aleurone cell layer, which is located between the endosperm and the outer bran layers, and in the scutellum(Reference Moore, Zhao and Gritsch8–Reference Wan, Stewart and Amrahli12). Up to 90 % of the iron in wheat is found in these regions. Zinc is more widely distributed in the plant and is found in scutellar epithelium, endosperm transfer cells and the embryonic axis(Reference Neal, Geraki and Borg9–Reference Wan, Stewart and Amrahli12). In contrast, the starchy endosperm is low in minerals. Therefore, when wheat is milled to produce white starch most of the mineral and vitamin content of the wheat grain is removed. The Bread and Flour Regulations (1998)(13) require some key minerals (iron and calcium) and B-vitamins (niacin, thiamine and more recently folic acid) to be added back into white wheat starch to compensate for the losses during processing.
Much of the iron and zinc in wheat co-localises with phosphorus, present as phytic acid (IP6; inositol hexakisphosphate; phytate) and this leads to the formation of mineral–phytate complexes(Reference Moore, Zhao and Gritsch8,Reference De Brier, Gomand and Donner10,Reference Wan, Stewart and Amrahli12) . In aleurone cells, iron and phytate are incorporated into phytin globoids inside the protein storage vacuoles, which potentially limits bioaccessibility during digestion(Reference Moore, Zhao and Gritsch8). Furthermore, the low solubility of mineral–phytate complexes may also reduce mineral bioavailability from cereal-based foods. In addition to phytate, zinc, particularly in the embryonic axis, may be associated with the thiol groups in proteins(Reference Persson, Hansen and Laursen14). The difference in the localisation and speciation of iron and zinc in cereals may explain the higher bioavailability of zinc (estimated to be 25 %) compared with iron (approximately 10 %) from wheat(Reference Bouis and Welch15).
A further limiting factor to mineral bioaccessibility from cereals is the presence of the plant cell walls, which are resistant to digestion in the human gastrointestinal tract. For example, aleurone cell walls are formed mainly of water-insoluble fibres including arabinoxylans and cellulose and remain largely intact during milling and food processing(Reference Brouns, Hemery and Price16,Reference Edwards, Grundy and Grassby17) . Several studies have shown that wheat cell walls are resistant to digestion in the small intestine and the nutrients encapsulated within intact wheat cells have low bioaccessibility(Reference Edwards, Grundy and Grassby17–Reference Latunde-Dada, Li and Parodi19). Our own work has shown that iron-rich aleurone cells transit undigested through the mouse gut and emerge intact in faeces(Reference Latunde-Dada, Li and Parodi19).
Mineral bioavailability
In order to be absorbed, minerals need to be liberated from food during digestion and presented in a water-soluble form that can be transported by proteins resident on the apical membrane of enterocytes, namely DMT1 (divalent metal transporter), for iron(Reference Gunshin, Mackenzie and Berger20,Reference Tandy, Williams and Leggett21) and ZIP4 (zinc import protein), for zinc(Reference Dufner-Beattie, Wang and Kuo22,Reference Liuzzi, Bobo and Lichten23) . Mineral bioaccessibility from foods ultimately determines mineral bioavailability. In the case of cereals, the high phytic acid content, physical encapsulation by plant cell walls and the removal of mineral-rich structures during food processing (e.g. milling to produce white starch) all affect bioavailability.
Effects of phytic acid
The bioavailability of iron is governed by several dietary factors (reviewed in(Reference Sharp24)), but the principal regulators of absorption are ascorbic acid, which acts as a reducing agent to maintain iron in its absorbable ferrous (Fe2+) form and stimulates absorption, and phytic acid which forms insoluble complexes with iron (and zinc) at intestinal pH and thereby inhibits absorption. Thus, the high phytate content in cereals decreases the bioavailability of iron and zinc. However, when consumed as part of a meal, foods containing elevated levels of ascorbic acid can, to some extent, counteract the inhibitory effects of phytic acid on iron absorption(Reference Hallberg, Brune and Rossander25).
Several food preparation strategies are known to be effective in reducing phytic acid levels in cereal foods. Wheat itself contains phytase enzyme activity which allows the liberation of phosphorus stored in phytic acid(Reference Bohn, Meyer and Rasmussen26). Grain particle size, fermentation time and hydrothermal processing have all been shown to influence phytate levels(Reference Majzoobi, Pashangeh and Farahnaky27). Furthermore, the addition of yeast, which is common in bread-making, introduces additional phytase enzyme activity and reduces levels of phytic acid in the final product(Reference Türk, Carlsson and Sandberg28). Sourdough fermentation reduces the pH of wheat doughs and activates endogenous wheat phytase activity reducing the phytate content of bread(Reference Leenhardt, Levrat-Verny and Chanliaud29). Studies comparing bread-making processes (sourdough v. conventional bread making v. Chorleywood bread process) have observed differential effects on phytic acid levels(Reference Rodriguez-Ramiro, Brearley and Bruggraber30). While levels of IP6 in the conventional and Chorleywood breads were more than 50 % below that seen in the wholewheat starch, levels of IP6 in the sourdough bread were below the levels of detection(Reference Rodriguez-Ramiro, Brearley and Bruggraber30). In this study(Reference Rodriguez-Ramiro, Brearley and Bruggraber30) and others where phytic acid levels in bread have been reduced by fermentation(Reference Brune, Rossander-Hultén and Hallberg31–Reference Sanz-Penella, Laparra and Sanz33) there was an associated increase in iron bioaccessibility and bioavailability.
Disruption of plant cell walls
Changes to food structure following thermal and mechanical processing can greatly influence nutrient bioaccessibility(Reference Edwards, Grundy and Grassby17,Reference Edwards, Ryden and Mandalari18,Reference Grundy, Edwards and Mackie34) . For example, in wheat, hydrothermal treatment causes swelling and gelatinisation of starch, which changes food structure and influences food digestion and nutrient absorption(Reference Edwards, Warren and Campbell35). Furthermore, addition of yeast during bread-making not only reduces phytic acid, but also results in changes to loaf volume and texture(Reference Struyf, Van der Maelen and Hemdane36). Leavening agents increase rates of digestion of starches(Reference Garcia-Hernandez, Roldan-Cruz and Vernon-Carter37), while natural fermentation (e.g. sourdough) of cereals can modify cellular structure through the extraction of phenolic compounds from cell walls(Reference Ravisankar, Dizlek and Awika38). We have investigated whether changes to the cellular structure of wheat might also influence mineral bioavailability. Enhanced mechanical disruption during milling to micronised starch particle size increases the bioaccessibility of iron, increases its solubility and enhances iron absorption in vitro (Reference Latunde-Dada, Li and Parodi19). The present paper measures iron and zinc bioavailability from breads made using the standard and micronised starch. Interestingly, enzymatic disruption of plant cell walls also increases iron bioaccessibility from wheat starch(Reference Latunde-Dada, Li and Parodi19). Thus, the use of food-grade enzymes during bread-making, for example, using xylanases to target the arabinoxylan-rich aleurone cell walls to increase iron release from its major storage site in cereals, might improve iron bioavailability. A similar approach has been shown to increase iron bioaccessibility from injera starch (a mix of teff, wheat and sorghum)(Reference Baye, Guyot and Icard-Vernière39).
Biofortification
Recognising the pressures on global food systems to produce sufficient nutrient-dense foods to feed the expanding global population, novel strategies have been introduced to increase nutrient content in staple foods. To date, this biofortification approach has focused on increasing iron, zinc and vitamin A content of foods commonly consumed in low- and middle-income countries (reviewed in(Reference Lockyer, White and Buttriss40)). This can be achieved through either agronomic practices, for example, the application of micronutrient rich fertilisers or sprays to increase nutrient content of the crops; conventional breeding to select for crops with higher levels of the nutrients of interest; or genetic methods to introduce or increase the expression of specific genetic traits that lead to enhanced accumulation of particular nutrients. The background to the development of biofortification as a strategy and its global reach in terms of populations consuming biofortified crops has been reviewed elsewhere(Reference Lockyer, White and Buttriss40).
Agronomic biofortification, where minerals are either applied to the soil in fertiliser or applied directly to the plant through foliar sprays, has proved effective in increasing the content of some minerals and trace elements. For example, the zinc content of rice(Reference Prom-U-Thai, Rashid and Ram41), maize(Reference Botoman, Chimungu and Bailey42) and wheat(Reference Signorell, Zimmermann and Cakmak43) can be increased by agronomic biofortification. In preliminary work using an in vitro digestion model, we have shown that increased zinc content in agronomically biofortified maize starch is bioavailable(Reference Watts, Aslam and Gunaratna44). Furthermore, Signorell et al.(Reference Signorell, Zimmermann and Cakmak43) in a human dietary intervention study, demonstrated the bioavailability of zinc from foods produced from agronomically biofortified wheat.
Conventional breeding has also been used successfully to develop iron and zinc biofortified staple crops. For example, iron-biofortified beans have increased iron bioavailability(Reference Petry, Rohner and Gahutu45). Subsequent studies have shown that consumption of biofortified beans improves iron status(Reference Haas, Luna and Lung'aho46) and is associated with improved cognitive performance(Reference Murray-Kolb, Wenger and Scott47) and physical work efficiency(Reference Luna, Pompano and Lung'aho48). Zincol-2016, a variety of zinc biofortified wheat has been developed and grown in Pakistan. In a short-term randomised controlled trial, consumption of foods incorporating Zincol-2016 starch was associated with increased zinc intake and elevated plasma zinc levels(Reference Lowe, Zaman and Khan49). Current work is evaluating zinc absorption from breads made in Pakistan from biofortified and control wheat in both randomised controlled trials and in vitro digestion studies(Reference Lowe, Zaman and Moran50).
Developing iron-biofortified cereals has proved to be more challenging due to the lack of variation in genetic traits associated with iron accumulation. Iron-biofortified pearl millet has been successfully introduced(Reference Cercamondi, Egli and Mitchikpe51), but so far, the development of iron-biofortified wheat remains elusive. Strategies to overcome this problem include a genetic biofortification approach in which a wheat vacuolar iron transporter (TaVIT2), which permits iron accumulation in aleurone cells, has been over-expressed in wheat under the control of an endosperm-specific promoter. This strategy has proved successful in targeting TaVIT2 to the endosperm and mobilising iron from the aleurone into the endosperm(Reference Connorton, Jones and Rodríguez-Ramiro52). Notably, the level of iron present in endosperm of the biofortified wheat is greater than that required through mandatory fortification of white starch suggesting potential nutritional benefits. An alternative approach has been to over-express the rice nicotianamine synthase gene (OsNAS2) in wheat(Reference Beasley, Bonneau and Sánchez-Palacios53). This enzyme increases the production of nicotianamine (NA), which is subsequently metabolised to deoxymugineic acid (DMA). Both NA and DMA are endogenous chelators of iron and zinc in cereals(Reference Eagling, Neal and McGrath54). This genetic strategy increases iron and zinc content in the grain of transfected wheat(Reference Beasley, Bonneau and Sánchez-Palacios53). More recently these two genetic strategies have been combined to produce a double-transfected wheat line (VIT2-NAS), which is high-yielding and contains elevated iron levels in endosperm and shows high iron bioaccessibility in in vitro digestion assays(Reference Harrington, Connorton and Nyangoma55). Interestingly, these mineral–NA and –DMA chelates are thought to be bioavailable and recently a specific intestinal iron–NA transport pathway has been identified, which utilises the amino acid transporter PAT1(Reference Murata, Yoshida and Sakamoto56). It is hoped that the iron bioavailability from breads made from this new VIT2-NAS wheat line will be tested soon in human dietary interventions.
While it is currently not possible to chemically synthesise NA due to its rare azetidine ring, some work is currently developing synthetic DMA derivatives for potential use in fertilisers(Reference Suzuki, Urabe and Sasaki57). If DMA from fertiliser is taken up by wheat and incorporated into the endosperm this has the potential to both increase the iron and zinc content of wheat and to hold these minerals in bioavailable forms in white wheat starch.
Fortification
The mineral content of wheat varieties used for bread-making varies greatly depending on the growth site, year of harvest, soil conditions, etc. Estimated wholegrain wheat iron levels are between 25 and 40 mg/kg dry weight and zinc levels between 15 and 35 mg/kg dry weight(Reference Wan, Stewart and Amrahli12,Reference Latunde-Dada, Li and Parodi19,Reference Harrington, Connorton and Nyangoma55,Reference Zhao, Su and Dunham58) . However, as most of the iron and zinc in wheat resides in the aleurone and embryo, respectively, and these structures are removed during milling to produce white starch, the final mineral content of white wheat starch is substantially reduced compared to the wholegrain starch (as low as 6–10 mg of iron and zinc/kg starch(Reference Zhao, Su and Dunham58)). The Bread & Flour Regulations (1998)(13) were introduced to compensate for nutrient losses resulting from production of white starch and require all white and brown wheat starch produced in the UK to be fortified with iron to a level that would be seen in wholemeal starch. This mandatory minimum level is set at 16⋅5 mg iron/kg starch. While the UK legislation allows for several forms of iron to be used to restore white starch levels, the most commonly used form is reduced elemental iron powder. This has the advantage of being inert so it does not affect the quality and shelf-life of bread and has no discernible adverse sensory properties (e.g. changes in colour, smell or taste). However, elemental iron is not soluble in water or dilute acid and therefore has low bioavailability compared with other iron compounds(Reference Hurrell59).
Several randomised controlled trials have investigated the efficacy of foods fortified with elemental iron on iron status. These trials have produced inconsistent results with several showing no benefit on Hb or serum ferritin levels (reviewed in(60)). Countrywide fortification programmes have also produced mixed results. For example, in Denmark, where fortification with carbonyl iron was discontinued in 1987, follow-up studies have found no increase in the prevalence or iron deficiency or iron deficiency anaemia in adult males or females(Reference Milman, Ovesen and Byg61,Reference Milman, Byg and Ovesen62) . In Sweden, where fortification was in place until 1994, discontinuation of white wheat starch fortification with iron has led to a 39 % decrease in dietary iron intake and an increase in the incidence of iron deficiency in adolescent girls, but not in adolescent boys(Reference Sjöberg and Hulthén63). The Scientific Advisory Committee on Nutrition in the UK has reviewed the Bread & Flour Regulations and the evidence for mandatory fortification of starch in the UK(64). Scientific Advisory Committee on Nutrition concluded that there was still a public health benefit to mandatory fortification with iron. If this process was discontinued it would increase the proportion of the population with low-dietary iron intakes, and this would disproportionally affect lower socio-economic groups.
While the data presented earlier relates to elemental iron, there is evidence that fortification with other forms of iron may be more efficacious. For example, a recent Cochrane Database systematic review and meta-analysis of trials with iron-fortified wheat starch (not restricted to elemental iron) found that there may be some benefit of fortification on the incidence of anaemia(Reference Field, Mithra and Peña-Rosas65). This raises the possibility that other novel fortification strategies could be considered.
In collaboration with partners at Bühler Industries we have investigated iron bioaccessibility and bioavailability of purified aleurone starch. The iron content of the aleurone starch is approximately four times higher than wholegrain starch (120–140 mg/kg dry weight) and in vitro assays suggest that the iron from aleurone starch is both bioaccessible and bioavailable(Reference Latunde-Dada, Li and Parodi19). We have hypothesised that addition of aleurone starch to white starch, at levels that would meet the mandatory fortification target in the UK, would provide a bioavailable source of iron for white bread.
Future challenges
In this review, we have highlighted several strategies that could increase mineral bioavailability from cereal products. Much of the work on mineral bioavailability has focused on white wheat starch products, particularly bread. UK Flour Millers estimate that bread is purchased by more than 90 % of households. However, despite many marketing campaigns highlighting the benefits of wholegrain foods, up to 70 % of all bread sold is white bread and only 10 % would be classed as wholegrain.
Biofortified crops, including cereals, offer great potential to increase mineral intakes globally. However, as yet they have not been tested for growth, yield and nutrient content in the UK. If adopted, they have the potential to improve mineral nutrition, but there would be challenges for the food industry and retailers to ensure that these foods made from these nutrient-dense crops are available at a price that is affordable to the lower socio-economic groups who would benefit most from their introduction. Genetic biofortification could hasten progress in the development of new cereal crops with enhanced nutritional properties, for example, the study by Harrington et al.(Reference Harrington, Connorton and Nyangoma55) has the potential to increase iron levels in white wheat starch. However, the use of these technologies to develop crops for human consumption is subject to legislation relating to GM organisms, and there remains opposition from some lobby groups and sections of the population to the production of GM foods.
To increase the nutritional properties of white starch with respect to minerals, we have proposed the use of aleurone as a potential food fortificant for use in white starch wheat products(Reference Aslam, Ellis and Berry66). Our preliminary work indicates that iron from aleurone is bioavailable(Reference Latunde-Dada, Li and Parodi19); however, the use of aleurone for widespread fortification would require changes to milling strategies and food production lines, which would have cost implications for industry and consumers. Interestingly, a similar approach has been proposed in which fava bean starch is incorporated into white bread(Reference Lovegrove, O'Sullivan and Tosi67). Current white bread production in the UK incorporates imported soya starch as a bread improver and the proposal is to replace soya with UK-grown fava bean starch. Importantly, fava beans also have high levels of iron and the incorporation of fava bean starch into white bread could increase the iron content of the standard loaf(Reference Lovegrove, O'Sullivan and Tosi67).
The use of alternative bread-making technologies has potential benefits for mineral nutrition. For example, sourdough processing reduces phytic acid levels and increases iron bioavailability(Reference Rodriguez-Ramiro, Brearley and Bruggraber30). The use of food-grade enzymes such as xylanases and phytases has the potential to increase both mineral bioaccessibility by disrupting plant cell walls and mineral bioavailability by decreasing phytate, but the use of these enzymatic approaches is still not widespread in industrial bread-making.
Cereal products remain the single biggest contributor to mineral intakes in the UK diet. They are particularly important in the diets of the adolescent population who are at risk of multiple micronutrient deficiencies. This review has shown that there are several viable strategies to increase mineral content and bioavailability from cereal products. To achieve substantial changes in mineral nutrition will require a food systems approach involving close collaboration with agronomists, farmers, food industry, retailers and consumers. However, there is a realistic prospect that at least one of the strategies discussed here could be developed in the coming years to improve the nutritional quality of cereal-based foods.
Conclusions
Globally, a high proportion of dietary iron and zinc is provided by cereal-based foods. However, the bioaccessibility and bioavailability of these minerals from cereals is limited by their localisation in the cereal plant tissue and the presence of high levels of phytic acid, the main dietary inhibitor of mineral absorption. Agricultural, food processing and culinary methods can potentially increase mineral bioavailability from cereal products. These methods include biofortification or conventional fortification to increase iron and zinc levels in cereals and foods; the disruption of plant cell walls to increase the liberation of nutrients from foods during digestion; and reduction of phytic acid levels during food preparation and cooking. Together these strategies could improve the nutritional quality of cereal-based foods and provide a solution to the low bioavailability of minerals in the diets of vulnerable populations.
Acknowledgements
The authors thank Walter von Reding (Bühler AG, Uzwil, Switzerland) and Simon Penson (ADM Milling, Corby, UK) for providing materials, and Michael Adams and Lucas Westphal (Campden BRI, Chipping Campden, UK) for food analysis, relating to some of the studies described in this review.
Financial Support
Some of the work described in this review was funded by the Biotechnology and Biological Sciences Research Council (grant numbers: BB/N021002 and BB/P017584). S. M. A. was supported by a PhD student scholarship from King Abdulaziz University, Jeddah, Saudi Arabia.
Conflict of Interest
None.
Authorship
The first draft of this review was written by P. A. S. All authors provided input into the development of the manuscript. All authors approved the final submitted version.