Obesity is considered to be one of the major risk factors related to insulin resistance onset(Reference Kopelman1). However, not all types of dietary fats appear to be as obesogenic and to produce insulin resistance. In fact, there is much evidence suggesting that the intake of n-3 fatty acids, especially EPA, produces some improvements in insulin resistance and obesity features in several models of obesity and diabetes in rodents(Reference Nobukata, Ishikawa, Obata and Shibutani2–Reference Lombardo, Hein and Chicco5). However, the ultimate mechanisms sustaining the protective effects of n-3 fatty acids are still unclear.
In this context, it is well known that adipose tissue is able to synthesise and secrete adipokines that can modulate insulin sensitivity both locally and in other organs(Reference Fantuzzi6). In fact, it has been hypothesised that the regulation of these adipokines by n-3 fatty acids could mediate their beneficial effects on insulin resistance. For example, it was recently reported that EPA administration increases the ability of adipocytes to produce adiponectin, which could contribute to the insulin-sensitising properties of this fatty acid(Reference Perez-Matute, Perez-Echarri, Martinez, Marti and Moreno-Aliaga4, Reference Flachs, Mohamed-Ali, Horakova, Rossmeisl, Hosseinzadeh-Attar, Hensler, Ruzickova and Kopecky7, Reference Neschen, Morino, Rossbacher, Pongratz, Cline, Sono, Gillum and Shulman8).
The recent identification of two new adipokines, visfatin and apelin, with an important potential role in insulin resistance, has led to investigation of the effects of n-3 fatty acids on their regulation. Visfatin was recently identified as a secreted protein expressed and regulated in adipose tissue with a predominant abundance in visceral fat depots(Reference Fukuhara, Matsuda and Nishizawa9). An insulin-like action of this adipokine was initially reported both in vivo and in vitro due to a specific binding of visfatin with the insulin receptor(Reference Fukuhara, Matsuda and Nishizawa9). However, other studies found that visfatin does not exert any insulin-mimetic effects(Reference Revollo, Körner and Mills10). It has been reported that expression and circulating levels of visfatin increase in obesity and diabetes(Reference Fukuhara, Matsuda and Nishizawa9, Reference Sandeep, Velmurugan, Deepa and Mohan11, Reference Zhang, Gottardo, Thompson, Powers, Nolan, Duffy, Marescotti, Avogaro and Doria12); however, there are some controversial results concerning these features(Reference Sun, Bishop, Khalili, Vasdev, Gill, Pace, Fitzpatrick, Randell, Xie and Zhang13, Reference Pagano, Pilon, Olivieri, Mason, Fabris, Serra, Milan, Rossato, Federspil and Vettor14).
Regarding apelin, it has been shown that this adipokine is up-regulated in rodents and man during obesity, and that a strong relationship exists between adipocyte-secreted apelin and insulin levels(Reference Boucher, Masri and Daviaud15–Reference Kralisch, Lossner, Bluher, Paschke, Stumvoll and Fasshauer17). In fact, plasma apelin concentrations have been associated with hyperinsulinaemia, and the insulin-deficient mice (streptotocin-treated) showed lower mRNA levels of apelin as compared with controls(Reference Boucher, Masri and Daviaud15). It has been hypothesised that over-production of apelin in the obese could be one of the last protective defences before the emergence of obesity-related disorders such as insulin resistance and type 2 diabetes(Reference Castan-Laurell, Boucher, Dray, Daviaud, Guigne and Valet18).
In addition to their effects on adipokines, it has been suggested that n-3 fatty acids could also exert their positive actions on insulin sensitivity by modifying the levels of GLUT (GLUT1 and GLUT 4) in muscle and adipose tissue(Reference Mori, Murakawa, Katoh, Hata, Yokoyama, Tajima, Ikeda, Nobukata, Ishikawa and Shibutani3, Reference Aas, Rokling-Andersen, Kase, Thoresen and Rustan19). Furthermore, n-3 fatty acids could also improve hyperglycaemia and hyperinsulinaemia by regulating the hepatic enzymes involved in glucose phosphorylation (glucokinase; GK) or dephosphorylation (glucose-6-phosphatase; G6Pase), which control glucose utilisation and production by this organ(Reference Seoane, Barbera, Telemaque-Potts, Newgard and Guinovart20, Reference Delarue, LeFoll, Corporeau and Lucas21).
In the present study, we investigated the effects of the n-3 fatty acid EPA on visfatin and apelin circulating concentrations and mRNA expression levels in lean and overweight rats, as well as the effect of EPA on muscle and adipose GLUT mRNA and liver GK and G6Pase activity. We demonstrate for the first time that EPA stimulates visfatin and apelin adipose tissue production, which could be involved in its properties to protect against the development of insulin resistance.
Experimental methods
Animals and treatment
Twenty-nine male Wistar rats (aged 6 weeks), obtained from the Centre of Applied Pharmacology (CIFA, Pamplona, Spain), were housed (two or three per cage) in a temperature-controlled room (22 ± 2°C) with a 12 h light–dark cycle. All experimental procedures were performed under protocols approved by the University Ethics Committee for the use of laboratory animals, according to the National and Institutional Guidelines for Animal Care and Use. Animals were distributed in four experimental groups: control, control-EPA, overweight and overweight-EPA groups. All animals were maintained in an adaptation period of 4 d on a chow diet (Rodent Toxicology Diet; B&K Universal, Hull, UK) and given deionised water ad libitum. After this period of time, control and control-EPA groups were fed a standard pelleted diet (Rodent Toxicology Diet; B&K Universal) containing 76 % of carbohydrates, 6 % of lipids and 18 % of proteins (1515 kJ/100 g (362 kcal/100 g)). On the other hand, the overweight and overweight-EPA groups were fed a cafeteria diet composed of the following items: pâté, chips, bacon, chocolate, biscuits and pelleted diet (2:1:1:1:1:1, by wt.)(Reference Perez-Echarri, Perez-Matute, Martinez, Marti and Moreno-Aliaga22). The composition of this diet was 9 % energy as protein, 29 % as carbohydrate and 62 % as lipid by dry weight. All the animals had ad libitum access to water and food during 5 weeks. Simultaneously, the control-EPA and overweight-EPA groups were daily treated by oral administration with highly purified EPA ethyl ester (1 g/kg animal weight) during 35 d, whereas the same volume of water was orally administrated to the control and overweight groups, as previously shown in other studies(Reference Mori, Murakawa, Katoh, Hata, Yokoyama, Tajima, Ikeda, Nobukata, Ishikawa and Shibutani3, Reference Kusunoki, Tsutsumi and Hara23, Reference Nobukata, Ishikawa, Obata and Shibutani24). These control and overweight groups without treatment with any other type of fatty acid (such as SFA with equal chain length as EPA or oleic acid) could be adequately considered as control groups in our study design, as previously published(Reference Perez-Matute, Perez-Echarri, Martinez, Marti and Moreno-Aliaga4), since it has been demonstrated that supplementation with some other fatty acids is able to modify adiposity and the circulating levels of biochemical and hormonal markers planned to be determined in the present study(Reference Mori, Murakawa, Katoh, Hata, Yokoyama, Tajima, Ikeda, Nobukata, Ishikawa and Shibutani3).
Body weight and food intake were measured daily. At the end of the experimental period, and after an overnight fast, animal were euthanised by decapitation. The collected gastrocnemius muscle, liver and visceral white adipose tissue (WAT) depots were frozen in liquid N2 and stored at − 80°C(Reference Perez-Matute, Perez-Echarri, Martinez, Marti and Moreno-Aliaga4).
RNA analysis and quantitative real-time polymerase chain reaction
Total RNA was extracted from liver, visceral adipose tissue and muscle, according to the Gibco Life Technologies procedure using Trizol (Life Technologies Inc., Grand Island, NY, USA). UV absorbance and integrity gels were used to estimate RNA. After this process, 5 μg RNA were treated with DNA-free™ (Ambion Inc., Streetsville, ON, Canada) in a total reaction volume of 24 μl during 30 min at 37°C. Of this product, 13 μl were retrotranscripted to cDNA. Reaction conditions of retrotranscription were as follows: 60 min at 37°C and 5 min at 95°C. For real-time PCR amplification, 9 μl cDNA per reaction were used.
TaqMan probes for rat apelin (Rn00581093_m1), visfatin/PBEF1 (Rn00822046_m1), GLUT1 (Rn00593670_m1), GLUT4 (Rn00562597_m1), insulin receptor (Rn00567070_m1) and 18S (Hs99999901_s1) were Assay-on-Demand gene expression products. All of the reagents for real-time PCR analysis of genes (TaqMan RT reagents and TaqMan Universal PCR Master mix) were purchased from Applied Biosystems (Foster City, CA, USA) and the conditions were used according to the manufacturer's protocol. Amplification and detection of specific products were performed with the ABI PRISM 7000HT Sequence Detection System (Applied Biosystems). 18S RNA was used as the reference to normalise the expression levels between samples, allowing data to be expressed relative to 18S RNA, therefore compensating for any differences in RT efficacy. All standards and samples were analysed in duplicate. Data were obtained as cycle threshold (Ct) values (the cycle at where the fluorescence signal emitted is significantly above background levels and is inversely proportional to the initial template copy number) according to the manufacturer's guidelines, and used to determine ΔCt values (ΔCt = Ct of the target gene − Ct of the housekeeping gene) of each sample. Fold changes of gene expression were calculated by the 2− ΔΔCt method(Reference Marrades, Milagro, Martinez and Moreno-Aliaga25).
Enzymic determinations
Liver GK activity was measured by spectrophotometrical detection of the NADH produced by the glucose-6-phosphate dehydrogenase (G6PDH)-induced dehydrogenation of glucose-6-phosphate in the presence of ATP and NAD+(Reference Newgard, Hirsch, Foster and McGarry26). The tissue (0·6 g in 10 ml of 50 mm 2-amino-2-hydroxymethyl-propane-1,3-diol (Tris)–HCl buffer, pH 7·4) was homogenised and subsequently centrifuged at 12 000 g for 60 min at 4°C, and then the supernatant fractions were collected. Then 10 μl of the supernatant fractions were incubated with 5·5 μl G6PDH and 985 μl of enzymic substrate (100 mm-glucose, 5 mm-ATP, 1 mm-NAD and 7·5 mm-MgCl2 in 100 mm-Tris–HCl buffer, pH 7·4) during 10 min in a temperature-controlled bath at 37°C. Samples were incubated at 4°C to stop the reaction, and then the absorbances were measured in a spectrophotometer at 340 nm(Reference Milagro, Gomez-Ambrosi, Forga and Martinez27).
Furthermore, liver G6Pase activity was quantified by measuring the phosphate released from the dephosphorylation of glucose-6-phosphate by G6Pase. Briefly, 0·5 g liver were homogenised in 4·5 ml 0·25 m-sucrose-HEPES buffer (pH 7·4) and subsequently centrifuged at 30 000 g for 10 min at 4°C. Then the supernatant fraction was centrifuged at 100 000 g for 60 min at 4°C. The pellet obtained was re-suspended again in the same buffer and the centrifugation was repeated for washing the pellet. This pellet, which contained the microsomes, was then re-suspended in 0·9 ml of the same buffer. A mixture of 100 μl of microsomes and 400 μl of reactive solution (1 mm-glucose-6-phosphate, 0·25 mm-CaCl2 and 1·25 mm-EDTA in 100 mm-Tris–HCl buffer, pH 7·3) was incubated at 37°C during 6 min in a temperature-controlled shaking bath. To stop the reaction, 2 ml ascorbic acid–TCA solution (2–10 %, w/v) were then added. The released phosphate was measured using the method of Fiske–Subbarow(Reference Baginski, Foa, Zak and Bergemeyer28) and expressed relative to the protein content in the homogenate(Reference Bradford29).
Serum visfatin and apelin determinations
Serum visfatin and apelin levels were measured by ELISA kits from ALPCO Diagnostics Ltd (Salem, NH, USA) and Phoenix Pharmaceuticals Inc. (Belmont, CA, USA) respectively, according to the manufacturers' guidelines.
Statistical analysis
Statistical differences and interactions were evaluated through a factorial two-way ANOVA (diet × EPA treatment). When statistically significant differences resulted at the interaction level, a Student's t test was carried out to compare the effects of each treatment (GraphPad Prism; GraphPad Software Inc., San Diego, CA, USA). Furthermore, Pearson correlation analysis was performed to screen potential association between two variables. Differences were considered as statistically significant at P < 0·05.
Results
Effects of eicosapentaenoic acid ethyl ester on body and fat pad weights and on glucose and insulin serum levels
The administration of EPA ethyl ester (1 g/kg) was able to partially prevent the body-weight gain induced by the cafeteria diet in rats (Table 1), although it did not reach statistical significance. Furthermore, EPA treatment tended to decrease the weight of all the fat pads, especially in the group of animals fed with a standard diet. In this sense, a significant reduction (P < 0·05) in retroperitonal adipose tissue weight was observed in EPA-treated rats. Although no significant changes were observed in serum glucose levels, EPA treatment induced a significant reduction (P < 0·05) in insulin plasma levels (Table 1). Moreover, insulin sensitivity was analysed by assessing the insulin:glucose ratio and the homeostatic model assessment (HOMA) index. The HOMA index was initially defined as an index of insulin resistance in humans(Reference Matthews, Hosker, Rudenski, Naylor, Treacher and Turner30). Even though HOMA index has not been validated for animal studies(Reference Wallace, Levy and Matthews31), several trials have demonstrated that the HOMA index can be useful to compare the resistance to insulin between groups in rodents(Reference Perez-Matute, Perez-Echarri, Martinez, Marti and Moreno-Aliaga4, Reference Milagro, Campión and Martínez32–Reference Ribot, Rodríguez, Rodríguez and Palou35). Our data revealed that both the insulin:glucose ratio and HOMA index were significantly decreased (P < 0·05) in EPA ethyl ester-treated rats, suggesting the ability of EPA supplementation to increase insulin sensitivity (Table 1).
Table 1 Effects of EPA ethyl ester (35 d of treatment) on body and fat weights, and on circulating levels of glucose, insulin and index of insulin resistance in lean and overweight rats*
(Mean values with their standard errors)

HOMA, homeostatic model assessment.
* Data were analysed by a two-way factorial ANOVA.
† HOMA = insulin (μUnits) × glucose (mmol/l)/22·5.
Effects of eicosapentaenoic acid ethyl ester on visfatin circulating and gene expression levels
A significant decrease (P < 0·01) in visfatin mRNA levels in visceral WAT was found in cafeteria-fed rats, which was reversed by EPA ethyl ester administration (P < 0·05) (Fig. 1 (a)). However, no significant changes were found in visfatin circulating levels (Fig. 1 (b)), despite the different size of fat depots exhibited by cafeteria-fed rats in comparison with EPA-treated animals. Adipose secretion of several adipokines, such as leptin and adiponectin, has been shown to be dependent on adipose mass size(Reference Havel36). In order to estimate the capacity of adipose tissue to produce visfatin and the involvement of visfatin from fat pads in the total blood concentration, serum levels of this adipokine were corrected for adiposity (visfatin per g total WAT). Then, a significant decrease in the vistatin:total WAT ratio (P < 0·05) was obtained in the cafeteria-fed groups (Fig. 1 (c)). In addition, our present data showed an inverse relationship between serum visfatin levels corrected for adiposity and HOMA index (r − 0·436; P < 0·05).
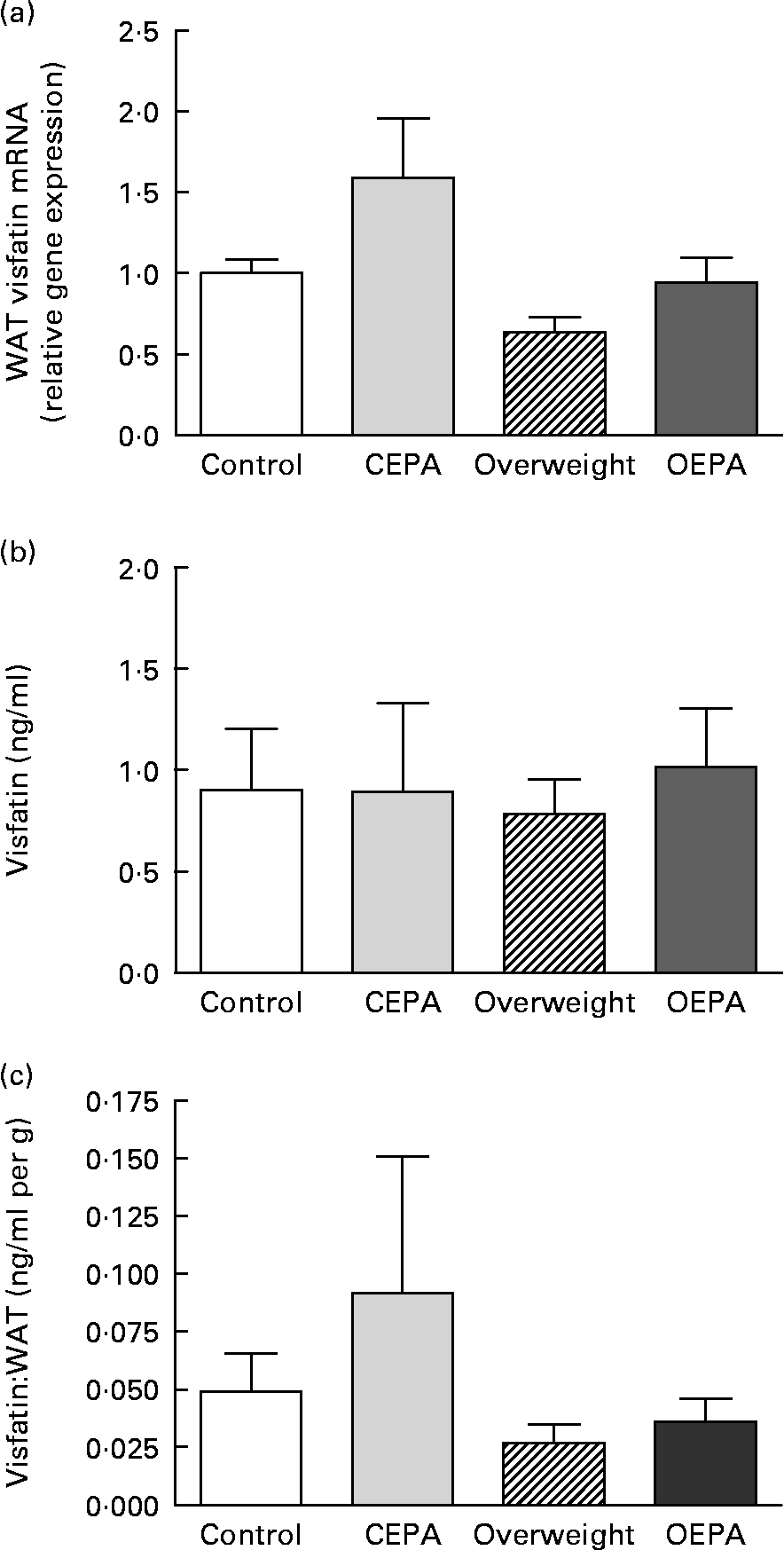
Fig. 1 Effects of EPA ethyl ester on visfatin gene expression and circulating levels in lean and overweight rats. (a) Visfatin mRNA expression levels in visceral white adipose tissue (WAT) obtained by real-time PCR. Data were calculated by the 2− ΔΔCt method, where Ct is cycle threshold. The mean value for the control group was set at 1 and 18S RNA was used as the reference to normalise the expression levels. The effect of diet was significant (P < 0·01), the effect of EPA treatment was significant (P < 0·05) and the interaction between diet and EPA treatment was NS. (b) Visfatin circulating levels. The effect of diet was NS, the effect of EPA treatment was NS and the interaction between diet and EPA treatment was NS. (c) Visfatin concentrations expressed per g WAT. The effect of diet was significant (P < 0·05), the effect of EPA treatment was NS and the interaction between diet and EPA treatment was NS. Data are means from at least seven independent animals per group, with standard errors represented by vertical bars. Data were analysed by two-way ANOVA. CEPA, control-EPA; OEPA, overweight-EPA.
Effects of eicosapentaenoic acid ethyl ester on apelin circulating and gene expression levels
In contrast to findings concerning visfatin, cafeteria-diet feeding caused a significant increase (P < 0·01) in apelin mRNA expression in visceral WAT. Similarly, the administration of EPA ethyl ester also induced a significant increase (P < 0·01) in apelin gene expression (Fig. 2 (a)). A similar pattern of increased apelin circulating levels in cafeteria-fed rats was observed, although no statistically significant differences were detected (Fig. 2 (b)). Furthermore, our data also showed that both apelin mRNA and the serum apelin:total WAT ratio were negatively correlated (P < 0·05) to the HOMA index (Fig. 2 (c) and (d)).

Fig. 2 Effects of EPA ethyl ester on apelin gene expression and circulating levels in lean and overweight rats. (a) Apelin mRNA expression levels in visceral white adipose tissue (WAT) obtained by real-time PCR. Data were calculated by the 2− ΔΔCt method, where Ct is cycle threshold. The mean value for the control group was set at 1 and 18S RNA was used as the reference to normalise the expression levels. Data are means from at least seven independent animals per group, with standard errors represented by vertical bars. Data were analysed by two-way ANOVA. The effect of diet was significant (P < 0·01), the effect of EPA treatment was significant (P < 0·01) and the interaction between diet and EPA treatment was NS. CEPA, control-EPA; OEPA, overweight-EPA. (b) Apelin circulating levels. Data are means from at least seven independent animals per group, with standard errors represented by vertical bars. Data were analysed by two-way ANOVA. The effect of diet was NS, the effect of EPA treatment was NS and the interaction between diet and EPA treatment was NS. (c) Correlation between mRNA apelin expression levels and homeostatic model assessment (HOMA) index (r − 0·3105; P = 0·0427). (□) Control; (![]() ), CEPA; (▲), overweight; (△), OEPA. (d) Correlation between serum apelin levels corrected for adiposity and HOMA index (r − 0·4645; P = 0·019).
), CEPA; (▲), overweight; (△), OEPA. (d) Correlation between serum apelin levels corrected for adiposity and HOMA index (r − 0·4645; P = 0·019).
Effects of eicosapentaenoic acid ethyl ester on mRNA levels of glucose transporters (GLUT-1 and GLUT-4) and insulin receptor
As shown in Table 2, no significant effects either by the cafeteria-diet feeding or by EPA administration were observed on GLUT1 and GLUT4 as well as on insulin receptor mRNA levels in gastrocnemious skeletal muscle. However, a significant decrease (P < 0·05) in GLUT-1 and GLUT-4 gene expression levels was found in visceral adipose tissue from EPA ethyl ester-treated rats, while no changes were induced in adipose tissue GLUT by the cafeteria-diet feeding (Table 2).
Table 2 Effects of EPA ethyl ester on mRNA expression levels of GLUT (GLUT-1 and GLUT-4) and insulin receptor (IR) in skeletal muscle (gastrocnemious) and visceral adipose tissue from lean and overweight rats*
(Mean values with their standard errors)

Ct, cycle threshold.
* Data were calculated by the 2− ΔΔCt method. 18S RNA was used as the reference to normalise the expression levels. The mean value for the control group was set at 1. Data were analysed by a two-way factorial ANOVA.
Effects of eicosapentaenoic acid ethyl ester on hepatic glucokinase and glucose-6-phosphatase
A significant increase (P < 0·05) in GK activity was observed in cafeteria-fed rats, while no significant changes were found in EPA-treated rats. G6Pase activity, as well as the GK:G6Pase ratio, which reflects hepatic glucose production, was not modified either by the cafeteria diet or EPA treatment (Table 3).
Table 3 Effects of EPA ethyl ester on liver glucose-6-phosphatase (G6Pase) and glucokinase (GK) activity, and on G6Pase:GK ratio in lean and overweight rats*
(Mean values with their standard errors)
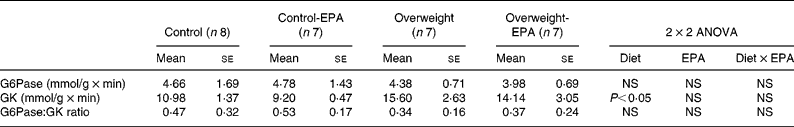
* Data were analysed by a two-way factorial ANOVA.
Discussion
In agreement with previous studies using different models of obesity and/or diabetes(Reference Mori, Murakawa, Katoh, Hata, Yokoyama, Tajima, Ikeda, Nobukata, Ishikawa and Shibutani3, Reference Perez-Matute, Perez-Echarri, Martinez, Marti and Moreno-Aliaga4, Reference Perez-Echarri, Perez-Matute, Martinez, Marti and Moreno-Aliaga22, Reference Kusunoki, Tsutsumi and Hara23), our data demonstrate the ability of EPA ethyl ester (1 g/kg) to improve insulin sensitivity. A primary goal of the present trial was to find out the potential role of adipose visfatin and apelin, two recently described adipokines, in the insulin-sensitising effects of EPA ethyl ester. Visfatin, which appears to be preferentially produced by visceral adipose tissue, is an adipokine with a controversial capacity to bind and activate the insulin receptor both in vivo and in vitro (Reference Fukuhara, Matsuda and Nishizawa9, Reference Revollo, Körner and Mills10). It has also been suggested that visfatin could be a missing link between intra-abdominal obesity and diabetes(Reference Fukuhara, Matsuda and Nishizawa9, Reference Sethi and Vidal-Puig37). However, controversial results about the role of visfatin in the regulation of glucose metabolism, insulin resistance and obesity situations have been recently described. Thus, although some studies have shown that serum and visceral fat visfatin mRNA levels are increased in obesity and type 2 diabetes(Reference Fukuhara, Matsuda and Nishizawa9, Reference Sandeep, Velmurugan, Deepa and Mohan11), visfatin gene expression in visceral adipose tissue of Wistar Ottawa Karlsburg W (WOKW) rats, a model of polygenic metabolic syndrome, was similar to that observed in lean control animals(Reference Klöting and Klöting38). Furthermore, some recent trials have even described that visfatin is down-regulated by overfeeding human subjects(Reference Sun, Bishop, Khalili, Vasdev, Gill, Pace, Fitzpatrick, Randell, Xie and Zhang13) and that circulating levels of visfatin are negatively correlated with visceral fat in humans genetically predisposed to develop insulin resistance(Reference Wang, van Greevenbroek, Bouwman, Brouwers, van der Kallen, Smit, Keijer and Mariman39). Pagano et al. (Reference Pagano, Pilon, Olivieri, Mason, Fabris, Serra, Milan, Rossato, Federspil and Vettor14) observed that plasma visfatin levels were reduced in human obesity. Our data suggest that overfeeding with a diet rich in saturated fat impairs visfatin gene transcription in visceral fat. The fact that cafeteria-fed rats exhibit similar serum levels of visfatin to the control rats, despite their higher adiposity, suggests that the ability of adipose tissue to produce visfatin is decreased in overweight animals. Indeed, the serum visfatin:total WAT ratio was significantly decreased in these cafeteria-fed animals. Longer periods of cafeteria feeding (more than 35 d) would probably be necessary to observe lower circulating levels of visfatin. A lack of concordance between visfatin circulating levels and gene expression in different fat depots has also been observed in other studies. Thus, Pagano et al. (Reference Pagano, Pilon, Olivieri, Mason, Fabris, Serra, Milan, Rossato, Federspil and Vettor14) found that in human obesity plasma visfatin levels are reduced, whereas visfatin mRNA was significantly higher in visceral fat, together with a down-regulation of the visfatin gene in subcutaneous fat. In contrast, Berndt et al. (Reference Berndt, Klöting, Kralisch, Kovacs, Fasshauer, Schön, Stumvoll and Blüher40) found that increased visfatin plasma concentrations in obesity correlate positively with visceral and negatively with subcutaneous visfatin mRNA levels. These facts suggest a complex divergent regulation of this adipokine in different fat depots. Moreover, visfatin is also expressed in skeletal muscle, liver and immune cells, whose function is also altered in obesity and it is likely that these tissues may also contribute to serum visfatin concentrations(Reference Pagano, Pilon, Olivieri, Mason, Fabris, Serra, Milan, Rossato, Federspil and Vettor14, Reference Samal, Sun, Stearns, Xie, Suggs and McNiece41).
Our data also demonstrate the ability of EPA ethyl ester to prevent the decrease of visfatin gene expression induced by the cafeteria diet. In this context, it has been described that visfatin increased after weight and adiposity loss induced by gastroplastic surgery(Reference Krzyzanowska, Mittermayer, Krugluger, Kopp and Schernthaner42). This finding suggests that the up-regulation of visfatin gene expression in visceral adipose tissue could be due to the reducing effects of EPA treatment on the size of this fat depot(Reference Perez-Matute, Perez-Echarri, Martinez, Marti and Moreno-Aliaga4). However, a direct stimulatory action of EPA on visfatin gene expression cannot be ruled out, similarly to what we have observed with leptin(Reference Pérez-Matute, Marti, Martínez, Fernández-Otero, Stanhope, Havel and Moreno-Aliaga43).
The physiopathological role of visfatin in the development of insulin resistance remains largely unknown(Reference Stephens and Vidal-Puig44). Some studies have suggested that visfatin is not related to insulin resistance in man(Reference Pagano, Pilon, Olivieri, Mason, Fabris, Serra, Milan, Rossato, Federspil and Vettor14). However, other studies have demonstrated that circulating levels of visfatin are negatively correlated with insulin resistance(Reference Wang, van Greevenbroek, Bouwman, Brouwers, van der Kallen, Smit, Keijer and Mariman39), and its plasma levels are decreased in diabetics compared with non-diabetic subjects(Reference Li, Yang, Li, Tang, Yang, Yang and Li45). The present study shows a negative correlation between the serum visfatin:total WAT ratio and HOMA index, supporting the idea that the increase in visfatin secretion could lead to an improvement in insulin resistance. In fact, our data suggest that the up-regulation of visfatin mRNA levels could contribute, at least in part, to the beneficial actions of treatment with the n-3 fatty acid EPA on insulin sensitivity. In this context, a recent study has shown that fatty acids such as oleic and palmitic acids may induce insulin resistance in 3T3-L1 adipocytes and preadipocytes, and that the down-regulation induced by these fatty acids on visfatin mRNA may contribute to impair insulin sensitivity(Reference Wen, Wang, Wu, Lu, Hu and Cianflone46). In addition, and in agreement with our trial, it was also described that the up-regulation of visfatin gene expression could be a mechanism through which PPAR-α, PPAR-γ and PPAR-δ agonists induced an improvement of insulin sensitivity in genetic obese and insulin-resistant Otsuka Long-Evans Tokushima Fatty (OLETF) rats(Reference Choi, Ryu, Lee, Kim, Seo, Kim, Kim, Choi, Baik and Choi47) and in Wistar rats fed on a high-fat diet(Reference Choi, Lee, Yoo, Ryu, Lee, Kim, Baik and Choi48). Moreover, it was described that the increase in circulating concentrations of visfatin after weight loss correlated with the decrease in plasma insulin concentration and HOMA of insulin resistance(Reference Krzyzanowska, Mittermayer, Krugluger, Kopp and Schernthaner42). It has also been demonstrated that the release of the adipocytokine visfatin is regulated by glucose and insulin both in vivo and in vitro (Reference Haider, Schaller, Kapiotis, Maier, Luger and Wolzt49). In fact, hyperinsulinaemia inhibits visfatin gene expression and secretion(Reference MacLaren, Cui and Cianflone50, Reference Garcia-Diaz, Campion, Milagro and Martinez51). These facts lead us to suggest that the reduced levels of basal insulin observed after EPA treatment(Reference Perez-Matute, Perez-Echarri, Martinez, Marti and Moreno-Aliaga4) could also contribute to the up-regulation of visfatin gene expression induced by EPA treatment.
Apelin was characterised as a novel adipose-expressed factor that increases during adipocyte differentiation, being also up-regulated in rodent and human obesity(Reference Boucher, Masri and Daviaud15, Reference Li, Yang, Li, Tang, Yang, Yang and Li45). Our experimental data revealed a significant increase in visceral apelin mRNA levels in cafeteria-fed rats. This is in agreement with a recent study that found positive associations between apelin gene expression levels in subcutaneous adipose tissue and the excessive weight gain induced by cafeteria feeding(Reference Garcia-Diaz, Campion, Milagro and Martinez51).
In addition, it was recently reported that the increase of some parameters involved in insulin resistance development, such as fat mass, insulin, glucose and lipid plasma levels and TNF-α gene expression, may positively influence apelin circulating levels by regulating its expression(Reference Li, Yang, Li, Tang, Yang, Yang and Li45, Reference Daviaud, Boucher and Gesta52). However, EPA treatment, which has been shown to decrease basal insulin concentrations, HOMA index and adipose TNF-α mRNA(Reference Perez-Matute, Perez-Echarri, Martinez, Marti and Moreno-Aliaga4) also induced a significant increase in apelin gene expression. Although a similar trend was observed for serum levels, no statistically significant changes were reached, probably because of the high variability of the data. Moreover, if EPA treatment were to last more than 5 weeks or if a higher dose were administered, it might be possible to observe significant changes in apelin, as well as in visfatin, levels.
In this sense, it has previously been hypothesised that overproduction of apelin in the situation of obesity could be one of the last protective defences before type 2 diabetes develops(Reference Castan-Laurell, Boucher, Dray, Daviaud, Guigne and Valet18). This idea suggests that the improvement in insulin resistance observed in EPA ethyl ester-treated rats could be associated, at least in part, to the increase in apelin gene expression and secretion by adipose tissue. Indeed, our data revealed a negative correlation between both apelin mRNA levels and the serum apelin:total WAT ratio with the HOMA index, supporting the relationship between insulin sensitivity and the amount of apelin produced by adipose tissue. In agreement with this idea, it was recently published that the intraperitoneal administration of apelin increased insulin sensitivity in vivo by influencing circulating adiponectin levels, the expression of brown adipose tissue uncoupling protein 1 and energy expenditure in mice(Reference Higuchi, Masaki, Gotoh, Chiba, Katsuragi, Tanaka, Kakuma and Yoshimatsu53).
On the other hand, it has been reported that the expression of GLUT1 and GLUT4 in WAT, as well as GLUT4 in skeletal muscle, is decreased by high-fat feeding and insulin resistance conditions(Reference Pedersen, Kahn, Flier and Kahn54–Reference Kahn and Pedersen56). In addition, it has been suggested that fish oils could prevent insulin resistance by the prevention of the decreased expression of GLUT4 in both skeletal muscle and adipose tissue(Reference Delarue, LeFoll, Corporeau and Lucas21, Reference Kahn and Pedersen56, Reference Peyron-Caso, Fluteau-Nadler, Kabir, Guerre-Millo, Quignard-Boulangé, Slama and Rizkalla57). However, in our experimental model of diet-induced obesity, we only found a tendency to decrease GLUT4 mRNA in skeletal muscle, although this was not statistically significant, and no changes in adipose tissue GLUT1 or GLUT4 mRNA levels were observed in cafeteria-fed rats. Moreover, in the present study the treatment with EPA-ethyl ester during 5 weeks did not induce any significant change in muscle GLUT. This is agreement with the study of Taouis et al. (Reference Taouis, Dagou, Ster, Durand, Pinault and Delarue58), which reported that Wistar rats maintained during 4 weeks with a high-fat diet rich in n-3 PUFA showed changes in muscle membrane phospholipids, without affecting muscle GLUT-4 content. However, a previous study from Mori et al. (Reference Mori, Murakawa, Katoh, Hata, Yokoyama, Tajima, Ikeda, Nobukata, Ishikawa and Shibutani3) showed that daily administration of EPA ethyl ester during 17–18 weeks caused a significant increase in GLUT4 mRNA in skeletal muscle. Furthermore, and contrary to what could be expected, the present study shows that EPA ethyl ester treatment decreases adipose tissue GLUT1 and GLUT4 mRNA. This could be a mechanism by which EPA prevents glucose entrance into adipocytes and, consequently, reduces the synthesis of new fatty acids in WAT and adiposity, effects mainly observed in the retroperitoneal fat pad(Reference Perez-Matute, Perez-Echarri, Martinez, Marti and Moreno-Aliaga4). In this context, Minami et al. (Reference Minami, Ishimura, Sakamoto, Takishita, Mawatari, Okada and Nakaya59) reported that the beneficial effect of EPA ethyl ester on insulin sensitivity seems to be related to a decreased abdominal fat accumulation. Thus, our data showed a significant reduction in the retroperitoneal fat pad ( − 25 %) mediated by EPA administration, an effect that could also contribute to improved insulin sensitivity. Taouis et al. (Reference Taouis, Dagou, Ster, Durand, Pinault and Delarue58) observed that GLUT4 mRNA was diminished in rats fed a high-fat diet enriched with n-6 fatty acids, while GLUT4 mRNA was unaffected in those enriched with n-3. These apparently controversial effects between different studies could be explained by the different durations of the high-fat feeding and the treatment with fish oils or only with highly purified EPA, the composition of the diet, and/or the strain and physiological conditions (normal, obese, diabetic) of rats used in these studies.
It has also been suggested that the protective effect of n-3 fatty acids on insulin resistance could be due to the suppression of both the activity and expression of G6Pase, leading to a decrease in excessive hepatic glucose output(Reference Delarue, LeFoll, Corporeau and Lucas21, Reference Daniele, Bordet and Mithieux60). Liver GK is a key enzyme involved in the regulation of glucose storage in hepatocytes(Reference Zulet, Barber, Garcin, Higueret and Martinez61). However, our experiments did not reveal any significant effect of EPA ethyl ester treatment on GK and G6Pase activity, as well as on the G6Pase:GK ratio (which roughly reflects hepatic glucose production), suggesting that the possible diminution in glucose production by the liver is not the major factor contributing to the improvement in insulin resistance in EPA ethyl ester-treated rats.
In conclusion, our data demonstrate the ability of EPA ethyl ester to prevent the decrease induced by the cafeteria diet in visfatin and apelin mRNA levels in visceral adipose tissue, and suggest that these effects could contribute, at least in part, to the insulin-sensitising effects of EPA. Our data also would indicate that changes in skeletal muscle GLUT, as well as in hepatic glucose production, are not likely to be major factors contributing to the improvement in insulin resistance after EPA ethyl ester treatment.
Acknowledgements
The present study has been supported in part by the Ministerio de Educación y Ciencia of Spain (AGL 2006-04716/ALI), by the Government of Navarra (Department of Education and Culture) and by Línea Especial de Investigación ‘Nutrición, Obesidad y Salud’ (University of Navarra/lE/97). N. P.-E. was supported by a doctoral grant from the Government of Navarra (Department of Education). The expert technical assistance of A. Lorente and V. Ciaurriz is gratefully acknowledged. EPA was generously provided by Brudy S.L. (Spain). There are no conflicts of interest. All authors listed have contributed to the work: N. P.-E. wrote the manuscript and performed the experimental work with the collaboration of P. P.-M. (animal treatment) and B. M.-G. (gene expression studies). J. A. M. participated in the experimental design and in the writing of the manuscript. M. J. M.-A. is the main investigator of the projects that support the present study; she participated in the experimental design, contributed to the data analysis and discussion, and the writing of the manuscript.







