INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) causes a high burden of disease, particularly in hospital settings [Reference Grundmann1]. Despite the active ‘Search-and-Destroy’ policy in The Netherlands [Reference Wertheim2], the burden of MRSA seems to have gradually increased over recent years, as reflected in the number of MRSA isolates obtained through screening, or from infected patients, which were sent to the national reference centre: <400 bacterial isolations in 2000 rising to 2693 in 2008 ([3], A. P. J. Haenen, unpublished data). However, when comparing S. aureus resistance rates in bloodstream infections in surrounding countries (Germany 20%, Belgium 21%, UK 31%, data for 2008 [4]), the methicillin-resistance rate of Dutch S. aureus isolates remains as low as 1%.
In 2003, a new type of MRSA (ST398) emerged in Dutch hospitals and has been found particularly in persons having contact with pigs or veal calves [Reference Huijsdens5–Reference Voss7]. Since these first notifications, this new type has been increasingly isolated [3, Reference Haenen8]. This ‘so-called’ livestock-associated (LA) MRSA was found in 39% of nasal samples obtained from 540 pigs sampled at nine Dutch slaughterhouses and represented 81% of positive farms [Reference de Neeling9]. In a subsequent study, LA-MRSA was detected on 56% of 50 pig farms investigated and in 29% of the pig farmers [Reference Van Den Broek10]. In a study on veal farms similar figures were obtained: LA-MRSA was found on 88% of veal calf farms (90/102), in 28% of veal calves (458/2151), and in 32% of the veal farmers (125/390) [Reference Graveland11]. In June 2006, the Dutch Working Party on Infection Control (www.wip.nl) adjusted the national ‘Search-and-Destroy’ policy to include routine screening for MRSA carriage in patients having regular contact with pigs or veal calves. As a consequence of this change in screening protocol, the proportion of LA-MRSA detected increased to 42% in 2008 (A. P. J. Haenen, unpublished data).
In a recent Dutch survey on MRSA in raw meat products sampled at retail, not only pork and veal was contaminated with MRSA (10·7% and 15·2%, respectively), but also a quarter of the fresh chicken samples (the majority with skin) of Dutch or EU origin were found positive [Reference de Boer12]. This meat was predominantly contaminated with LA-MRSA ST398 (85%). Two recent Belgian studies found limited circulation of ST398 in poultry: 12% of 81 S. aureus strains isolated from broilers in 2006 [Reference Nemati13], and 2/14 MRSA-positive broiler farms [Reference Persoons14]. In a 2001 Korean survey MRSA was isolated from 3/296 poultry samples; once in a retail meat sample and twice from chicken with arthritis [Reference Lee15]. Similar low prevalences have been reported from Japan [Reference Kitai16], Jordan [Reference Quddoumi17], and Spain [Reference Lopez18]. The first time LA-MRSA was found on a Dutch poultry farm was in 2006, and was isolated from a patient working on this farm [Reference Leenders19]. In a subsequent small survey on three additional poultry farms, MRSA was found in one environmental sample from the stables and in 5/6 adults living on these farms, but in none of the three children.
Therefore, between November 2008 and July 2009, we conducted a study on MRSA in Dutch broiler slaughterhouses. The primary aim of this study was to estimate the prevalence of MRSA in Dutch broiler flocks at the moment of delivery at the slaughterhouse. Other study endpoints were (i) to determine the degree of MRSA contamination in the different compartments of the slaughterhouse at the beginning and the end of a working day in order to gain insight in transmission and risks of infection for personnel working at the slaughterhouse; (ii) to estimate the risk of MRSA carriage in personnel, and (iii) to gain insights into the transmission mechanism of MRSA to humans at broiler slaughterhouses.
METHODS
Study population and questionnaires
Through the Association of Dutch Poultry Processing Industries (NEPLUVI), which are together responsible for 90% of production, eight of the larger slaughterhouses were approached to participate in this prevalence study. Six agreed to participate. Their individual daily production varied between 85 000 and 175 000 broilers, and their total daily production was around 775 000 broilers, which is about 45% of the national production. The annual production of broilers in The Netherlands for 2008 was 451 545 100 broilers (data from Statistics Netherlands; www.cbs.nl), carried out at 55 slaughterhouses. The slaughterhouses were situated at various locations, predominantly in the east and south-east of The Netherlands, with the exception of one, which was located in the north-west. The information collected on each participating slaughterhouse included number of employees, slaughterhouse capacity, specifics on lairages and the production process, information on microbiological contamination of the birds and working surfaces as well as hygiene control measures. The actual design of the production process is automated and standardized to a large degree and as such did not differ much between the slaughterhouses. However, a few differences were observed in the degree of automation: e.g. at one slaughterhouse the racks containing crates of broilers were automatically placed from the lorries onto a conveyor belt, and crates were again automatically emptied onto the next conveyor belt before broilers were stunned. As a result of this automation, almost no personnel were working in the delivery area. Furthermore, two methods of stunning were used: a relatively modern method of CO2 stunning, and a more common method of electric stunning using a waterbath. Birds were hanged on the slaughterline after CO2 stunning or before electric stunning.
According to the Dutch guidelines on MRSA control (www.wip.nl), ⩾2% MRSA positive is considered a significant increase from the expected background value of 0·5% in The Netherlands. The required sample size was calculated at 450 employees (α=0·05 and β=0·10) [Reference Lwanga20]. The survey of personnel contained questions on age, gender, ethnic origin, recent antibiotic treatment, job description, job rotation between different sections of the slaughterhouse, contact with live animals professionally and/or privately, contact with family members working in healthcare, e.g. nursing homes or hospitals, or in livestock farming. Slaughterhouse workers were divided in three main categories according to their activities in the production process: (1) contact with live broilers (not necessarily the only activity), (2) only contact with dead broilers and meat products and (3) other, i.e. administrative and technical personnel. Participation of slaughterhouse personnel, as well as transporters, quality control personnel, and local employees of the Food and Consumer Product Safety Authority (VWA), was on a voluntary basis. Written consent was obtained from each participant.
Sample collection
At each slaughterhouse, a nasal swab (Venturi Transystem, Copan Innovation, Italy) was taken from both anterior nares of each human volunteer by samplers. The samplers themselves were sampled at the beginning and immediately after their activities as well as the following morning.
To determine the extent of MRSA prevalence in broilers, at each of the six slaughterhouses we randomly collected samples from 40 slaughter flocks originating only from 40 Dutch broiler farms. Geographic location of the supplying broiler farm was recorded. A total of 405 pharyngeal samples were taken from broilers immediately after stunning by the VWA veterinarians using common sterile dry cotton swabs (Greiner Bio-One B.V., The Netherlands). At four slaughterhouses, five slaughter flocks were sampled, and at two slaughterhouses 10 flocks. From each flock, ten animals were sampled. Sample collection from flocks mostly took place on the same day as human and environmental samples were taken, but also took place on different days, depending on the delivery date of the flocks. Additionally, at five slaughterhouses, surface swabs were taken from inside five or ten transport crates from each broiler flock. A flock was classified as positive if at least one MRSA sample was positive, either from chicken or crate.
Environmental samples using Sodibox wipes (Raisio Diagnostics B.V. Nieuwerkerk aan den IJssel, The Netherlands) were taken from surfaces from five different compartments in the six participating slaughterhouses. From each slaughterhouse 20 wipe samples in total were collected at the beginning and 20 at the end of the working day: three in the delivery area, six in the dirty area, seven in the processing area, two in the cooling area, and two in the cutting area, respectively.
Microbiological examination
At the Radboud University Nijmegen Medical Centre (RUNMC), nasal swabs from human volunteers and samplers were incubated in Mueller–Hinton enrichment broth (Becton Dickinson, USA) supplemented with 6·5% NaCl, for 18–24 h at 35°C. Next 10 μl of the supernatant was plated onto a MRSA-ID culture plate (bioMérieux, France), and incubated overnight at 35°C. Suspect (green) colonies were tested for antibiotic resistance. Isolates from animals and environment were tested at the Central Veterinary Institute for 12 antimicrobial agents with the Vitek system (bioMérieux SA, France) according to the manufacturer's instructions. Human isolates were tested for in vitro antibiotic resistance at the Centre for Infectious Disease Control Netherlands (CIb) according to the cefoxitin disc diffusion method [21]. Cefoxitin-resistant colonies were inoculated on Columbia agar plates with 5% sheep blood and incubated for 18–20 h at 35°C. A latex agglutination test (Staphaurex Plus; Murex Diagnostics Ltd, UK) was performed to confirm S. aureus. The MRSA isolates obtained were subsequently stored at −80°C.
Processing of environmental and animal samples was carried out at the CIb. Pre-enrichment of the pharyngeal swabs from broilers and of the environmental samples was carried out by transferring the swabs to 10 ml Mueller–Hinton enrichment broth (BBL, 211 443) with 6·5% NaCl and incubating for 18 h at 37°C, while the wipes were soaked in 100 ml broth. For selective enrichment of the MRSA, 1 ml of the broth was transferred into 9 ml of Phenol Red mannitol broth with 5 mg/l ceftizoxim and 75 mg/l aztreonam (bioMérieux) and incubated for 18 h at 37°C. Subsequently, 10 μl of the suspension was transferred onto a Columbia agar plate with 5% sheep blood (Oxoid, UK). In parallel, Brilliance MRSA culture plates (Oxoid) were inoculated with 10 μl suspension and incubated for 18 h at 37°C. Colonies were streak-plated onto sheep blood agar until pure colonies were obtained.
All MRSA isolates were genetically characterized by PCR specific for S. aureus [Reference Martineau22], the mecA gene [Reference de Neeling23], and the Panton–Valentine leucocidin (PVL) toxin genes [Reference Lina24]. Staphylococcal protein A (spa) typing was conducted according to Harmsen et al. [Reference Harmsen25]. A selection of isolates was further typed by multi-locus sequence typing (MLST) [Reference Enright26]. Finally, bacterial strains thus obtained were tested for antimicrobial susceptibility by broth microdilution according to ISO 20776-1:2006 with the antibiotics listed in Figure 1. For classification of resistance EUCAST interpretive criteria were used (www.eucast.org).
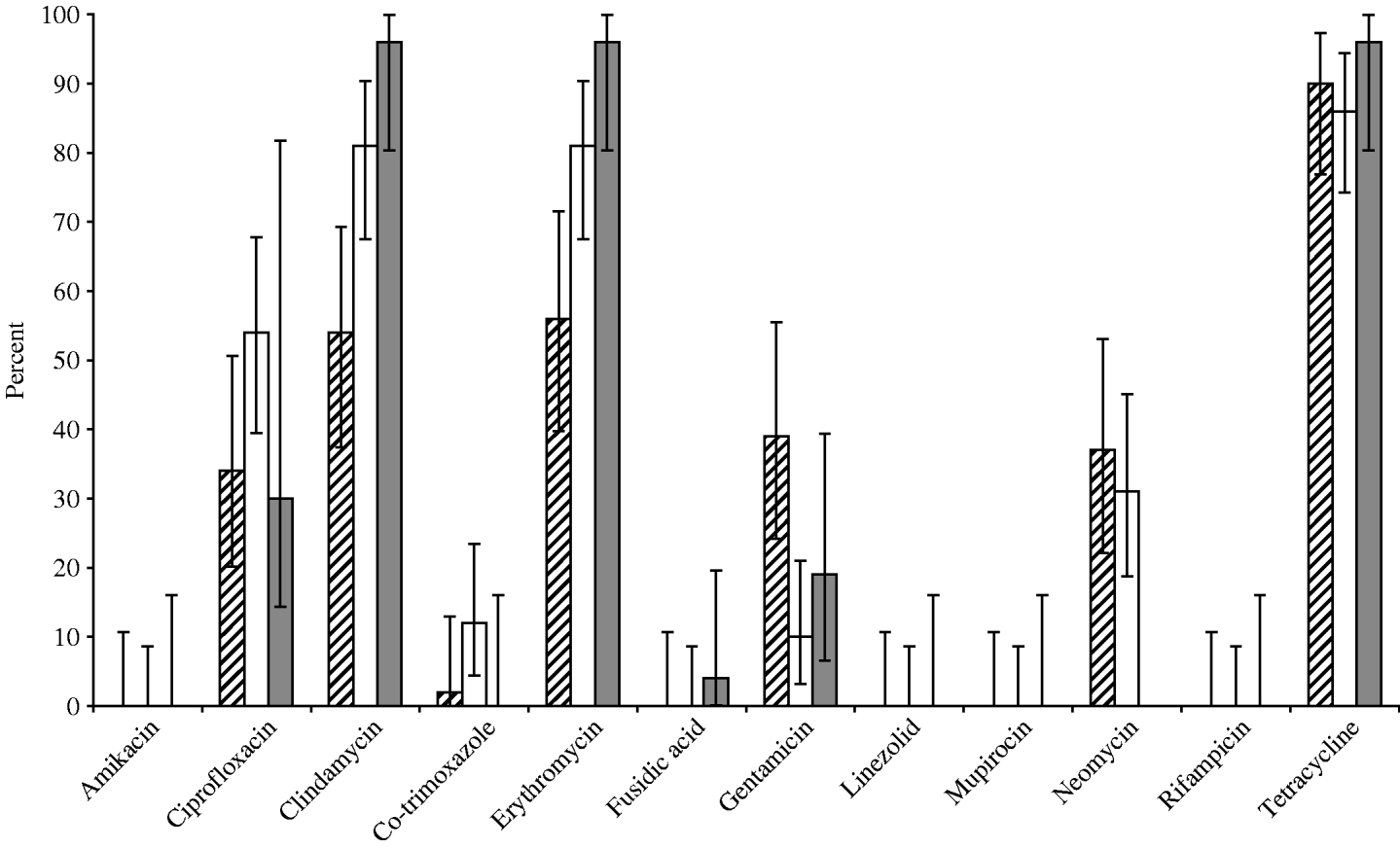
Fig. 1. Susceptibility for 12 important antibiotics of MRSA strains isolatesd from human volunteers (grey bars, 26 isolates), compartments within the slaughterhouse (white bars, 52 isolates) and broilers/transport containers (hatched bars, 41 isolates).
Statistical analysis
Data collected from the questionnaires were entered into a database and analysed using the statistical software package SAS version 9.1 (SAS Institute, USA). Risk factors for employees being MRSA positive were first identified by univariable logistic regression. A random slaughterhouse effect was included in the model to adjust for the fact that observations of humans at the same slaughterhouse might not be independent (proc glimmix). A multivariable multilevel analysis was performed by stepwise, forward entry of all factors with P<0·2 in the univariable analysis. Confounding was checked for by monitoring the change in regression parameters; confounding was considered to be present if parameters changed by >25% or, if case parameters were between −0·4 and 0·4, parameters changed by >0·1. Collinearity of independent variables was assessed based on the standard errors of the parameter estimates, but was not present in the analysis. In the final multivariable model interaction terms were tested for significance (P<0·05). The fit of the model was assessed with the Hosmer & Lemeshow goodness-of-fit-test (proc logistic). The exact confidence intervals (CIs) for prevalences were calculated based on the binomial probability function (proc freq). To correct CIs for clustering effect, e.g. for results of chickens within flocks, a Generalized Estimating Equations (GEE) model [Reference Liang27] was performed (proc genmod) with flock as a random effect. Cohen's kappa statistic for classifying a flock as positive or negative based on either results of chickens or crates was calculated (proc freq) [Reference Fleiss28].
MRSA prevalence in broilers was estimated according to Thrusfield [Reference Thrusfield29]. Geographical distribution of the supplying broiler farms was compared to the regional density distribution of broiler farms in The Netherlands (www.cbs.nl). A probable difference between regions was tested using a generalized linear model.
RESULTS
Descriptive statistics: prevalence in human volunteers
The total number of employees was 1006, all of whom were invited to participate. The response rate was 46% (n=466). The overall carriage rate of MRSA in poultry slaughterhouse workers was 5·6% (n=26; exact 95% CI 3·7–8·1, the 95% CI corrected for clustering effect within slaughterhouse is 2·6–11·4). The highest rate was found in the group of workers having contact with live broilers: 13·8% (19/138, exact 95% CI 8·5–20·7, varying between 0% and 25% in the different slaughterhouses; Table 1). People having contact only with dead animals have a lower carriage: 1·8% (4/224, exact 95% CI 0·5–4·5). In the group of ‘other’, which includes administrative and technical personnel, 2·1% were carrying MRSA (2/95; exact 95% CI 0·3–7·4). The remaining nine people did not indicate their main activity; one of them was a carrier (11·1%, 95% CI 0·3–48·3).
Table 1. Prevalence of MRSA isolated from specimens obtained from human volunteers at six Dutch slaughterhouses
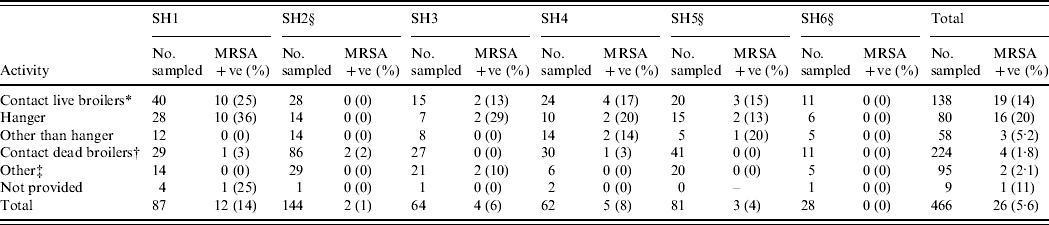
SH, Slaughterhouse.
* Contact live broilers: lorry drivers transporting live broilers, personnel working in reception, hanging, stunning/killing/bleeding, official veterinarian, official auxiliary.
† Contact only dead broilers: own quality inspectors, personnel working in scalding/plucking, cutting, packing.
‡ Other personnel: administrative, technical, canteen, forwarding, cleaning of crates, cold store, cold store lorry drivers.
§ CO2 stunning (other slaughterhouses use electric stunning).
When assessing the risk of MRSA acquisition depending on the method of stunning, the overall carriage of MRSA in personnel in the slaughterhouses using conventional electric stunning vs. CO2 was 9·9% (21/213, exact 95% CI 6·2–14·7) and 2·0% (5/253, exact 95% CI 0·6–4·6), respectively (Table 1). When assessing specifically the persons having contact with live broilers we found in slaughterhouses using conventional electric stunning an average carriage rate of 20·3% (16/79, exact 95% CI 12·0–30·1, varying between 13% and 24% per slaughterhouse). In slaughterhouses using CO2 the average prevalence in this subgroup was 5·1% (3/59, exact 95% CI 1·1–14·2, varying between 0% and 15% per slaughterhouse).
One of the five samplers became positive at the end of the working day with spa type t034, which was not isolated from broilers, the environment, or human volunteers from the same slaughterhouse, but which is one of the most common LA-MRSA types in The Netherlands. The next day this person was again negative, as before sample collection.
Risk factors human exposure
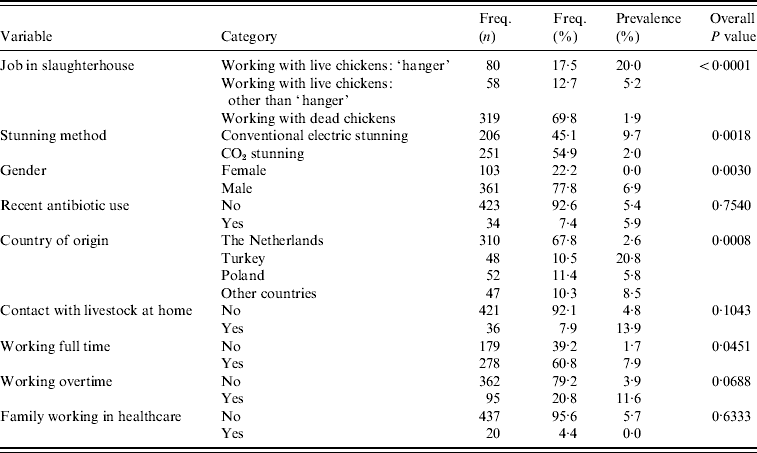
The association between MRSA presence in slaughterhouse employees was first investigated with a multilevel (random slaughterhouse effect) univariable logistic regression analysis. In this analysis the following risk factors showed an overall value of P<0·20 (Table 2). Employees for whom a job description was missing (n=9) were excluded from the analysis. Type of job within slaughterhouse (working with live broilers as ‘hanger’, working with live broilers other than ‘hanger’, working with dead broilers, P<0·0001), stunning method (conventional electric vs. CO2 stunning, P=0·0018), working overtime (yes/no, P=0·07), full-time job (yes/no, P=0·05), contact with livestock animals at home (yes/no, P=0·10), country of origin (Dutch, Turkish, Polish, other; overall P=0·0008), and gender (female/male, P=0·003). Of the employees with known gender, none of the females were positive (0/103, exact 95% CI 0–3·5) compared to 6·9% of the males (25/361, exact 95% CI 4·5–10·1); only two females worked with live birds. Therefore, gender could not be analysed in the multivariable model where type of job was included. Of the employees, 68% (310/457) were of Dutch origin, from which 2·6% were MRSA positive (exact 95% CI 1·1–5·0); other ethnicities testing positive were Polish (5·8%, 3/52, exact 95% CI 1·2–16·0), Turkish (20·8%, 10/48, exact 95% CI 10·5–35·0), and other nationalities (8·5%, 4/47, exact 95% CI 2·4–20·4). Of the Dutch employees, 11% worked as ‘hangers’, compared to respectively 44% (Turkish), 21% (Polish), and 32% (other) of non-Dutch employees. Recent, i.e. within the last 3 months, use of antibiotics of employees was not a significant risk for being MRSA positive (P=0·75), nor was having family working in healthcare (P=0·63).
Table 2. Univariable analysis accounting for clustering effect of slaughterhouse (proc glimmix)
In the multivariable analysis, two independent risk factors remained significant: type of job within the slaughterhouse and method of stunning (Table 3). People working with dead broilers had the lowest prevalence (1·9%). Compared to this group, people hanging broilers on the slaughterline were more often MRSA positive (20·0%, OR 11·27, 95% CI 4·18–30·43, P<0·0001) as well as people working with live broilers other than hanging broilers (5·2%, OR 2·26, 95% CI 0·54–9·46, P=0·27). The second risk factor was the method of stunning used in the slaughterhouse: people working in a slaughterhouse with conventional stunning have a higher prevalence of being MRSA positive (9·7%) than personnel working in slaughterhouses with CO2 stunning (2·0%) (OR 4·36, 95% CI 1·55–12·26, P=0·0053). The interaction effect was not significant (P=0·51). The effect of slaughterhouse was small as estimates were almost similar in the model with and without correction for the effect of slaughterhouse (Table 3). The Hosmer & Lemeshow goodness-of-fit statistic of the model without the random slaughterhouse effect was not significant (P=0·8305), indicating no lack of fit of the model.
Table 3. Multivariable analysis accounting for clustering effect of slaughterhouse (proc glimmix)
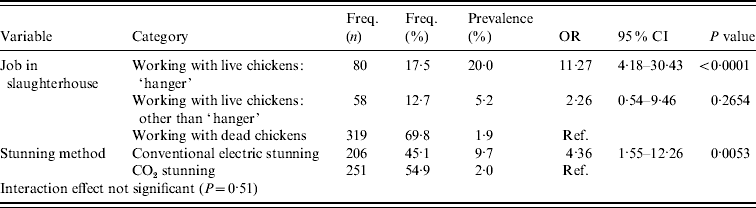
OR, Odds ratio; CI, confidence interval.
Broilers
In total, 405 broilers from 40 Dutch flocks were sampled randomly, 28 broilers were MRSA positive (6·9%, exact 95% CI 4·6–9·8), varying from 0% to 24% per slaughterhouse (Table 4). Furthermore, 9/40 flocks were positive based on broiler samples (22·5%, 95% CI 10·8–38·5). Of the flocks with positive broilers, one flock had 1/10 birds positive, six flocks had 2/10 positive, one flock had half positive, and in only one flock were all broilers MRSA positive. The difference in contamination of slaughter flocks was statistically significant (P<0·0001), which indicates a correlation between the broilers within flocks. Adjusting for this clustering effect (GEE [Reference Liang27]), the prevalence of MRSA-positive broilers was 6·9% (95% CI 3–14·9). Of the 151 transport containers, 13 were positive (8·6%, exact 95% CI 4·7–14·3; 0–20% per slaughterhouse). Of the flocks with positive crates (8/30), three flocks had only one wipe out of five collected that was positive, and five flocks had two wipes positive (Table 4).
Table 4. Prevalence of MRSA in broilers sampled at the slaughterhouseFootnote *
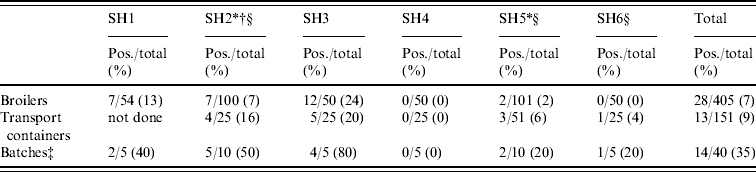
SH, Slaughterhouse.
* Sample collection from broilers and transport containers had not necessarily taken place on the same day when slaughterhouse compartments and human volunteers were sampled.
† The transport containers of five batches were sampled.
‡ Slaughter batch was considered positive when at least one container or animal was MRSA positive.
§ CO2 stunning (other slaughterhouses use electric stunning).
Of the 40 slaughter flocks thus sampled, 14 were MRSA positive and had either only broilers (n=6) or only crates (n=5), or both (n=3) positive. Cohen's kappa statistic of classifying a flock (n=30) based on either broilers or crates was 0·26, indicating a poor agreement. MRSA prevalence of positive broiler flocks was estimated as 35·0% (exact 95% CI 20·6–51·7). No specific geographic preference could be determined for MRSA prevalence at farm level.
Environment
Overall, 10 of the total 120 (8%, exact 95% CI 4·1–14·8) environmental samples collected at the beginning of the working day were MRSA positive (Table 5). At the end of the working day, this number increased to 42 (35%, exact 95% CI 26·5–44·2).
Table 5. MRSA prevalence in the different compartments within slaughterhouses both at start and end of working day
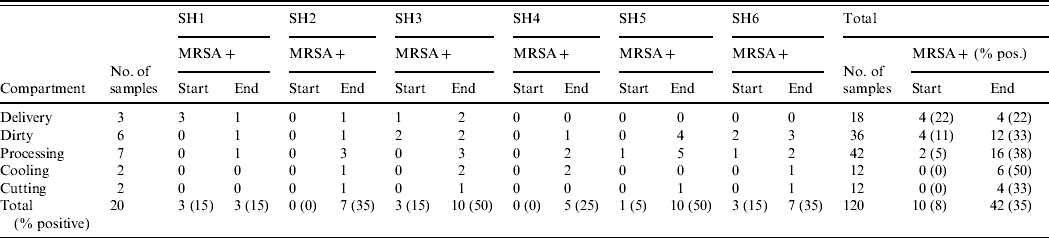
SH, Slaughterhouse.
In half of the slaughterhouses the delivery area remained MRSA negative throughout the day, whereas two were already positive at the start. In all six slaughterhouses the dirty area (where broilers are stunned, hanged on the slaughterline and slaughtered) became MRSA positive at the end of the day, two were already positive at the beginning. The same was true for the processing area: where at the beginning of the working day only 5% (2/42; exact 95% CI 0·6–16·2) of wipe specimens were positive (two halls positive), at the end of the day this percentage increased to 38% (16/42 samples, exact 95% CI 23·6–54·4, all halls became positive). The cooling areas were all negative at the start of the day, and four became positive during the day. The cutting areas were also all negative at the start, and in four slaughterhouses they became MRSA positive during the working day. In 5/6 slaughterhouses MRSA prevalence increased during the working day. Although two were MRSA free at the beginning, all became positive at the end of the day (15–50% of all wipes positive). At the beginning of the day the areas at the beginning of the slaughter process were mostly positive (particularly the dirty area), at the end MRSA was found throughout the whole slaughterline.
Microbiological characterization
In total, 119 MRSA strains were collected. These have all been microbiologically characterized. All strains were PVL negative. Based on their spa type, the strains predominantly belonged to the livestock-associated clonal complex MRSA-CC398. Six MRSA spa types have been found in the 26 human isolates (Table 6). The most predominant spa type was t011 (58%, n=15). Other spa types included t034 (n=3), t238 (n=1), t1430 (n=4), t1456 (n=1), t4652 (n=2). The spa types found in the 41 broiler isolates were also predominantly t011 (68%, n=28), but also t034 (n=1), t108 (n=4; ST398), and t1430 (n=8) were found. In the slaughterhouse, t1430 was the most commonly found spa type (n=20), next to t011 (n=16), and t034 (n=14), as well as two sporadic isolations of t108 (n=1) and t1456 (n=1). In the slaughter flocks where both transport crates and broilers were positive, the same spa types were isolated (t011, combination of t011/t1430, and t108). In three slaughter flocks a mix of spa types t011 and t1430 was found (http:// spaserver2.ridom.de/). Spa types t011, t034, t108 and t1456 all belonged to the livestock-associated MLST-type ST398. Additionally, one strain with spa type t4652 belonged to MLST ST1453, which is a new single locus variant of ST398; another strain of spa type t1430 was typed ST9; and the single isolate t238 belonged to ST1454. All spa types, except t238, and t1430 belonged to MRSA clonal complex CC398.
Table 6. Results spa-typing MRSA isolates obtained during the course of this study
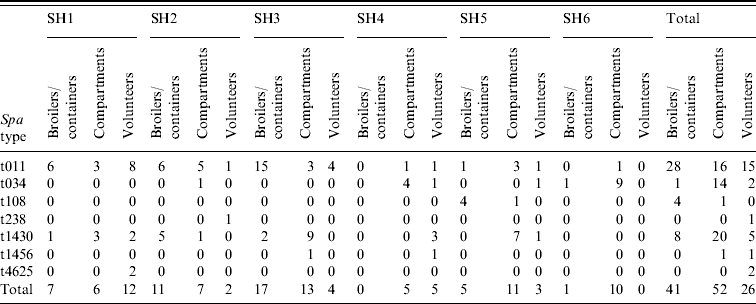
SH, Slaughterhouse.
Overall in vitro antibiotic resistance was found particularly for clindamycin (76%), erythromycin (76%) and tetracycline (89%, Fig. 1), as well as ciprofloxacin (42%), gentamicin (22%), and neomycine (26%), irrespective of the source of the isolates, broilers/transport crates, the environment or human volunteers. Additionally, low levels of resistance for co-trimoxazole were found in broilers (2%) and the environment (12%), but none in humans. The antibiotic resistance was particularly diverse in isolates belonging to spa types t011 and t1430 (Fig. 2). Co-trimoxazole resistance was only found in MRSA bacteria belonging to spa type t1430, whereas gentamicin resistance was solely found in spa types t011 and t1456. All spa type t034 isolates were multi-resistant for clindamycin, erythromycin and tetracycline, except one, which was only resistant for erythromycin (the only spa type t034 isolated from a transport crate). In total, 76% of the isolates (91/119) had multiple resistance against three or more antibiotics, predominantly (97%) a combination of clindamycin, erythromycin and tetracycline. Furthermore, 57% of all isolates were resistant to at least four different antibiotics. None of the strains were resistant against the other important antibiotics mupirocin, linezolid, or rifampicin.
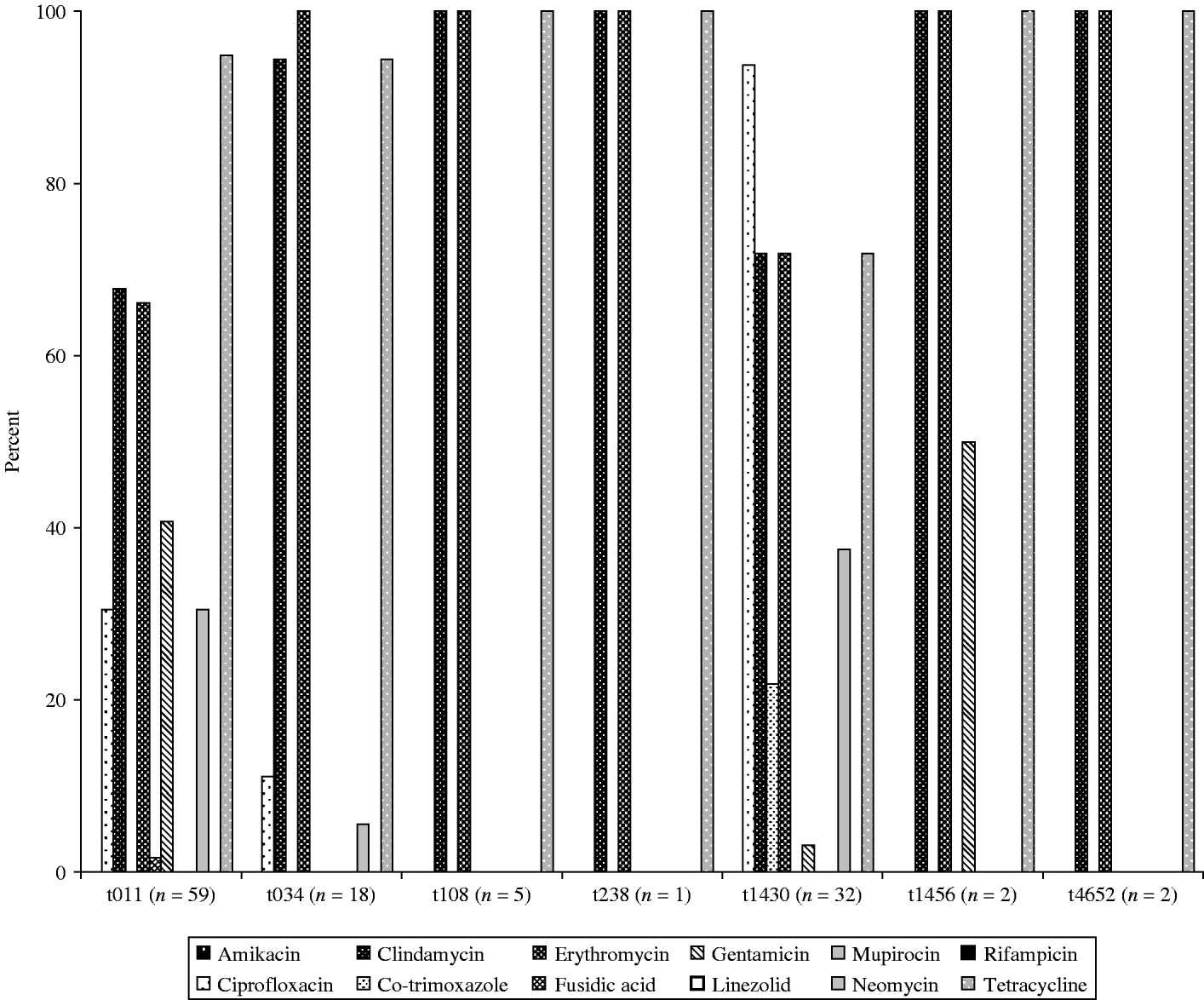
Fig. 2. Susceptibility for the 12 most common antibiotics of the seven different spa types found in the six slaughterhouses participating in the study.
DISCUSSION
The 5·6% prevalence of MRSA in personnel working at slaughterhouses found in our study seems to be higher than the estimated baseline MRSA prevalence of about 0·1% in the Dutch general population [3]. As such, there is an increased risk of exposure to MRSA when working at a broiler slaughterhouse. This risk is significantly larger for personnel having regular contact with live birds compared to persons having contact only with dead birds. In particular, hanging broilers from the slaughterline could be associated with an increased risk for MRSA carriage (20·0%) as could working with live chickens other than hanging (5·2%) compared to working with dead birds (1·9%). A similar prevalence of 15% in persons having contact with live animals was found in a comparable study, recently conducted in Dutch pig slaughterhouses (B. A. G. L. Van Cleef et al., unpublished data). MRSA could not be detected in persons working in other compartments of the slaughterhouses. Our finding of an increased risk of MRSA positivity associated with slaughterhouses using conventional electric stunning compared to CO2 stunning is striking. When electric stunning is used, birds are placed on the slaughterline alive prior to stunning, which might cause more dust in the environment as we observed extensive flapping of wings. If broilers are stunned with CO2, they are hanged only after stunning and wing flapping did not occur. Dust has been identified previously as a vector of MRSA transmission from animals to humans [Reference Van Den Broek10]; however, no measurements of the effect of wing flapping on MRSA transmission have been made in our study.
With respect to MRSA spread within the slaughterhouse, the cleaning and disinfection measures seem to be generally sufficient to control the spread of the bacterium. At the beginning of the working day, MRSA contamination of the different compartments in the slaughterhouses was relatively low, particularly in the ‘clean’ areas (processing, cooling and cutting areas). However, during meat processing, MRSA spreads along the slaughterline, as indicated by the overall increase in MRSA-positive environmental wipe samples obtained at the end of the working day. This increase of bacterial contamination along the slaughterline and during the production process has been shown previously to occur for other bacteria, e.g. Salmonella [Reference Rasschaert30], and Campylobacter [Reference Genigeorgis31]. Overall, 35% of the 40 slaughter flocks, which originated from 40 different Dutch broiler farms, were MRSA positive based on the presence of at least one positive animal in the specific flock or positive transport crate, or a combination of both. In total, 6·9% of the broilers were MRSA positive. Although transport crates are thoroughly cleaned and disinfected after each use, predominantly to control the spread of Salmonella, there always remains a slight chance that crates remain contaminated with MRSA, as a result of which MRSA prevalence in broiler flocks may be overestimated. Based on the geographic location of the 40 broiler farms, we could not establish a preferential distribution pattern of MRSA-positive farms. In comparison, a similar study in pig slaughterhouses found a much higher prevalence of 81% in slaughter flocks [Reference de Neeling9], and 39% of all pigs (n=540) were positive with LA-MRSA. Apparently, MRSA seems to be currently less widespread in the Dutch poultry population.
The majority of MRSA strains belonged to ST398, which is also the most commonly isolated LA-MRSA from humans in The Netherlands [Reference Haenen8]. The strains obtained from personnel working at the slaughterhouse belonged to a spa type also found in broilers and the environment. However, there was some inconsistency between the spa types isolated from the broilers and the environment, and the human volunteers. This could be explained by the fact that only Dutch flocks were sampled. Most slaughterhouses indicated that slaughter flocks from Germany were also processed. The partial concordance between the spa types of the strain isolated from broilers and humans and the mere fact that almost all of these spa types were LA-MRSA (only a single HA-MRSA found in one volunteer) is a strong indication that transmission of LA-MRSA from broilers to poultry workers occurs, either directly or indirectly through the working environment. The most frequently isolated spa type (t011) is also the most predominant in pigs [Reference Van Den Broek10] and veal [Reference Graveland11]. It is also the predominant spa type found in Dutch humans infected with LA-MRSA [Reference Haenen8]. The same spa type was also found in Belgian poultry [Reference Nemati13]. The frequent isolation of spa type t1430 from samples obtained from human volunteers, broilers and the environment is marked. This spa type does not belong to MLST type ST398 but to ST9, which are genetically unrelated. The same spa type t1430 was also found in a recent study conducted by the Dutch VWA in which 6·7% of retail chicken meat contained this particular spa type [Reference de Boer12]. These findings suggest that spa type t1430 is a specific poultry-associated MRSA type. Furthermore, according to recent studies [Reference Armand-Lefevre32–Reference Wagenaar35] ST9 has been found in livestock, suggesting that this MLST type is also livestock-associated MRSA. In that study two new STs were found. ST1453 is the fourth variant of ST398 found in The Netherlands, after ST752, and ST753.
The antibiotic resistance patterns we found in the various isolates from humans, broilers, and the environment are similar to the patters found in recent Belgian studies [Reference Nemati13, Reference Persoons14]. In contrast, however, in broiler isolates we found a high degree of resistance against ciprofloxacin. In the poultry industry (fluoro-)quinolones (most predominantly flumequin and enrofloxacin) are frequently used and may explain the high level of ciprofloxacin resistance. Since, we did not measure actual antibiotic usage, this is merely a hypothesis. The resistance pattern in general also corresponded with that in MRSA isolated from pigs, although all the porcine MRSA strains were sensitive to ciprofloxacin [Reference de Neeling9].
During this study we did not sample the finished meat products. Our findings on MRSA prevalence in broilers and the slaughterhouse environment are similar to results from a previous study on prevalence of MRSA in retail poultry meat, where a similar high rate was observed [Reference de Boer12]. The levels of contamination in terms of bacterial counts were very low. Based on these findings and epidemiological data it was concluded that MRSA-contaminated meat products play a negligible, if any, role in the spread of MRSA in the human population.
Previous studies on MRSA prevalence on pig farms [Reference Van Den Broek10] and veal farms [Reference Graveland11] showed high prevalences in humans. The hospital guidelines were subsequently modified: upon admission to a hospital, patients having regular contact with veal calves or pigs must be screened for MRSA and be confined to quarantine until carriage has been excluded. The degree of carriage in broiler farmers has not yet been determined. However, the results of this study prompted us to initiate a study on the prevalence in broiler farmers.
In conclusion, our study indicates that slaughterhouse personnel having contact with live broilers are at an increased risk for MRSA carriage. As the poultry industry involves a larger cohort who have contact with live poultry, including poultry farmers, their family members and employees, follow-up studies are needed to assess the degree of exposure to MRSA, to understand the risk factors involved, and to develop guidelines for intervention.
ACKNOWLEDGEMENTS
The authors thank the directors and personnel of the six slaughterhouses, the executive board of NEPLUVI, Suzanne Lamers and Emile Spalburg for laboratory testing, Brigitte van Cleef for developing the sampling protocols, Jan Muilwijk, Katsuhisa Takumi, Jan van de Kassteele for statistical analysis, and Han de Neeling for expert advice.
DECLARATION OF INTEREST
None.










