Introduction
Bovine anaplasmosis is a highly transmissible tick-borne disease that affects cattle and other ruminants (Watthanadirek et al., Reference Watthanadirek, Chawengkirttikul, Poolsawat, Junsiri, Boonmekam, Reamtong and Anuracpreeda2019), primarily caused by Anaplasma marginale. The disease is endemic in tropical and subtropical regions worldwide, causing significant health issues and economic losses in the livestock industry. Other species of Anaplasma, such as A. centrale, A. bovis and A. phagocytophilum, also cause various forms of the disease in cattle (Ybañez and Inokuma, Reference Ybañez and Inokuma2016). Transmission of Anaplasma species occurs through biological vectors (ticks), mechanical vectors (biting flies, fomites) (Radostits and Done, Reference Radostits and Done2007; Kocan et al., Reference Kocan, de la Fuente, Blouin, Coetzee and Ewing2010; Aubry and Geale, Reference Aubry and Geale2011) and rarely through the placenta (Van Loo et al., Reference Van Loo, Pascottini, Hooyberghs, De Bleecker, Ribbens, Opsomer and Pardon2023). Approximately, 20 tick species have been reported as vectors of A. marginale globally (Radostits and Done, Reference Radostits and Done2007); however, Rhipicephalus microplus was identified as a main natural vector in Bangladesh (Roy et al., Reference Roy, Krücken, Ahmed, Majumder, Baumann, Clausen and Nijhof2018). Biological vectors can maintain and propagate A. marginale for a significant length of time, making them crucial for disease transmission. However, some strains of A. marginale may rely on rapid mechanical transfer due to the limited quantity of the agent transferred (Kocan et al., Reference Kocan, de la Fuente, Blouin, Coetzee and Ewing2010; Aubry and Geale, Reference Aubry and Geale2011).
Anaplasmosis is clinically characterized by showing general weakness, weight loss, fever, severe anaemia, pale mucous membranes, abortion, lethargy, icterus, decreased milk production and often death in animals older than 2 years (Kocan et al., Reference Kocan, de la Fuente and Cabezas-Cruz2015). The severity of the disease depends on some factors, such as the host's immunological state and the presence of other pathogens (Constable et al., Reference Constable, Hinchcliff, Done and Grunberg2017). Recovered cattle may develop persistent infection which is considered an important epidemiological factor for bovine anaplasmosis. It has been observed that recovered cattle from acute cases, even those that have been treated with the recommended doses of tetracycline, continue to maintain a microscopically undetectable parasitaemia for their entire lives (Palmer et al., Reference Palmer, Brown and Rurangirwa2000; Radostits and Done, Reference Radostits and Done2007; Kocan et al., Reference Kocan, de la Fuente, Blouin, Coetzee and Ewing2010; Aubry and Geale, Reference Aubry and Geale2011). Persistently infected cattle that are exposed to mechanical and/or biological vectors can act as reservoirs of infection to introduce A. marginale into naive cattle populations (de Echaide et al., Reference de Echaide, Knowles, McGuire, Palmer, Suarez and McElwain2001; Futse et al., Reference Futse, Ueti, Knowles and Palmer2003).
Bangladesh has around 25.7 million cattle, demonstrating the importance of dairy and meat production in the country (World Bank, 2018). Bovine anaplasmosis has a severe economic impact on the dairy industries by reducing weight gain, milk and meat production, abortion, icterus and even death (Hove et al., Reference Hove, Khumalo, Chaisi, Oosthuizen, Brayton and Collins2018; Okafor et al., Reference Okafor, Collins, Daniel, Harvey, Sun, Coetzee and Whitlock2018). Several studies have been carried out previously on the subclinical and clinical bovine anaplasmosis in Bangladesh (Samad et al., Reference Samad, Bashar, Shahidullah and Ahmed1989; Talukder and Karim, Reference Talukder and Karim2001). A higher frequency of subclinical anaplasmosis (33%) was detected in the milk vita region of Sirajganj district (Talukder and Karim, Reference Talukder and Karim2001). On the other hand, 70% anaplasmosis was detected in cattle with possible clinical signs (Chowdhury et al., Reference Chowdhury, Hossain, Barua and Islam2006), and 1% prevalence of haemoprotozoan parasites was reported in Red Chittagong Cattle of Chattogram district (Siddiki et al., Reference Siddiki, Uddin, Hasan, Hossain, Rahman, Das, Sarker and Hossain2010), 22.74% in Sylhet (Akter et al., Reference Akter, Paul, Syed, Mandal and Nazneen2018) and 18.67% in Sirajganj (Islam et al., Reference Islam, Rahman, Hossain and Haque2019) based on the microscopic examination of Giemsa-stained blood smear. About 43% prevalence of bovine anaplasmosis was detected in Dhaka (Hassan et al., Reference Hassan, Giasuddin, Rahman, Ershaduzzaman and Hasan2019), 15.75% in Chattogram (Mannan et al., Reference Mannan, Alim, Manik, Mahbub, Rahman and Alamgir2022) and 82.86% in Bandarban (Mohanta et al., Reference Mohanta, Chikufenji, Galon, Ji, Ma, El-Sayed, Amer, Do and Xuan2023) through polymerase chain reaction (PCR). Although several epidemiological studies have been performed on bovine anaplasmosis in different regions of Bangladesh based on the microscopic examination of Giemsa-stained blood smears and PCR, the seroprevalence of bovine anaplasmosis has not yet been addressed in Bangladesh. The competitive enzyme-linked immunosorbent assay (cELISA) test is advised for population monitoring and screening, whereas PCR and microscopic examination of blood smears are advised for the investigation of clinical cases, according to diagnostic assays used in veterinary medicine for the detection of A. marginale and A. centrale. Currently, the prevalence of bovine anaplasmosis and its economic consequences have become a concerned issue in the country. Climate change, vector diversity and diverse geographical areas are making the situation more critical to control disease transmission and prevention. Knowledge regarding the local or regional prevalence of bovine anaplasmosis is required for the effective implementation of control strategies. In order to execute effective management programmes of bovine anaplasmosis in Bangladesh, it is imperative to determine the seroprevalence, which may serve as a lookout for estimating the prevalence of the disease in the study area.
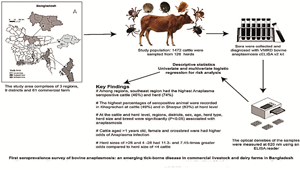
The present study investigated the seroprevalence of bovine anaplasmosis in commercial dairy farms in the northeast, central and southeast regions of Bangladesh using cELISA to provide comprehensive data to the scientific community for future planning to control the disease. The geographical locations of the 3 zones are characterized by plain, hilly and riverine areas. Therefore, the present study was conducted for the first time in Bangladesh to monitor the health status of livestock animals to detect the presence of Anaplasma infections by serological assay.
Materials and methods
Study area
The study was conducted in 3 dairy intensive regions of Bangladesh, viz., northeast, central and southeast regions (Fig. 1), from October 2022 to March 2024. The northeast region includes Mymensingh, Sherpur, Jamalpur and Netrakona districts, whereas the southeast region comprises Chattogram and Khagrachari districts and Dhaka, Gazipur and Narsingdi districts belong to the central region, respectively. These selected districts were promising for crossbreed dairy farming because of the growing demand for food derived from animals, the high density of the cattle population, the great potential for productivity enhancement, the agro-ecological conditions that support the production of feed, the accessibility of crop residues and the option of mixed crop–livestock farming (World Bank, 2018). The study area had more than one-third of the cattle farms of Bangladesh combined (Huque and Khan, Reference Huque and Khan2017) and the list of dairy farms was obtained from sub-district (Upazila) livestock offices posted in the respective district. The list of farms (sampling frame) from these districts of Bangladesh was entered into a spreadsheet (Microsoft Excel 2010). Each farm was assigned an Excel-generated random number using the ‘rand’ function, and 61 farms were selected. Then the herds were randomly selected from the sampling frame (Islam et al., Reference Islam, Rumi, Kabir, Van der Zanden, Kapur, Rahman, Ward, Bakker, Ross and Rahim2020). The farms with 2 cattle and at least 1 mature cattle were considered as an inclusion criterion for this study. All animals on the farm were included in the study, including weak and emaciated animals, with the exception of calves under 6 months of age and those in advanced pregnancy (>8 months). Geographic coordinates of each selected cattle farm were captured during blood sample collection using a handheld global positioning system reader (Garmin eTrex 10) (Islam et al., Reference Islam, Rumi, Kabir, Van der Zanden, Kapur, Rahman, Ward, Bakker, Ross and Rahim2020). ArcGIS-ArcMap version 10.3 (Environmental System Research Institute, Redlands, CA, USA) was used to visualize the spatial distribution of the cattle farms included in this study (Rahman et al., Reference Rahman, Sumon, Khan and Islam2015).

Figure 1. Map of the study districts of Bangladesh. A total of 61 cattle farms of 9 districts were surveyed; black circles are the GIS coordinates of selected farm.
Calculation of sample size and sampling procedure
The sample size was determined by Cochran's sample size formula (Cochran, Reference Cochran1977) for categorical data for an α level a priori at 0.05 (error of 5%), n 0 = (t)2*(p)(q)/(d)2 [where: n 0 is the sample size; t is the value for the selected α level, e.g. 1.96 for (0.25 in each tail) a 95% confidence level; p (5%) is the expected proportion of an attribute that is present in the population; q is 1−p; (p) (q) are the estimates of variance; d is the acceptable margin of error for the proportion being estimated, so the confidence interval, in decimals). A total of 1472 blood samples (552 samples from the northeast, 442 from the southeast and 478 samples from the central regions) were obtained from 61 commercial dairy farms in these 3 study regions.
Blood collection
Prior to collection of blood samples, farm owners' oral consent was obtained. From each cattle, 8 mL of blood was withdrawn via jugular venipuncture with disposable needles and 6 mL of blood was put into serum collection tubes, labelled and transferred to the laboratory of the Department of Parasitology, BAU, on ice (after clotting) within 12 h. Sera were extracted 1 day later by centrifuging at 3000×g for 30 min, after which blood samples were kept refrigerated (2–8°C) in the laboratory. Each serum sample was labelled with the animal's identification number and stored at −20°C.
Serological study using cELISA
All serum samples (1472) were analysed for the presence of Anaplasma-specific antibodies using a commercially available cELISA kit (Veterinary Medical Research and Development Inc., Pullman, WA, USA) according to the manufacturer's instructions and published literature (Parvizi et al., Reference Parvizi, El-Adawy, Melzer, Roesler, Neubauer and Mertens-Scholz2020). The wells of the ELISA plates were coated with Anaplasma spp. antigen provided with the commercial kits. The commercial kits included both positive and negative control sera for this assay. The optical densities of the samples were measured at 620 nm using an ELISA reader. The inhibition per cent was computed as I% = 100 (1 [sample OD620/OD620 of the negative control]) to understand the results. Any sample with <30 and ⩾30% was considered negative and positive, respectively. The manufacturer states that the test has >99% specificity and sensitivity with this cut-off. If a single animal was positive for Anaplasma infection, we considered the herd as positive (Islam et al., Reference Islam, Rumi, Kabir, Van der Zanden, Kapur, Rahman, Ward, Bakker, Ross and Rahim2020).
Data management and analysis
Animal, farm-level data and laboratory findings were entered into a spreadsheet (Microsoft Excel 2010). The dataset was coded, checked, validated for integrity and exported to SPSS Statistics software®, which was used to analyse the data (IBM Corp., Armonk, NY, USA, version 25). We calculated the mean and standard deviation (s.d.) for continuous variables and calculated proportions and frequency distributions for categorical variables. All continuous predictor variables [herd size, age of the animal, sex, breed and cattle raised for various purposes (calf, dairy heifer, beef heifer, bull and dairy cows)] were categorized prior to logistic regression analysis. Initially, univariable mixed-effects logistic regression analyses were performed to find out the effect of individual risk factors on Anaplasma infection. The variables that were statistically significant (P < 0.05) in the univariate analysis were selected as potential candidates for the multivariable analysis to find out the interaction of different variables. A backward stepwise elimination approach was applied in the multiple logistic regression. Variables with a P value <0.05 were retained in the final mixed-effects logistic regression model. Collinearity among explanatory variables was assessed by Cramer's phi-prime statistic and a pair of variables was considered collinear if Cramer's phi-prime statistic was >0.70 (Rahman et al., Reference Rahman, Islam, Talukder, Hassan, Dhand and Ward2017).
Results
Descriptive epidemiology
A total of 1472 dairy cattle from 61 randomly selected dairy farms, with a herd size of 126 (interquartile range, IQR), across 3 regions were sampled in the investigation. Overall, 42.93% of the cattle were recorded as seropositive in the study. The majority of the sampled cattle (37.5%) were from the northeast region, followed by the central region (32.5%) with the southeast region contributing the smallest proportion (30%) (Table 1). The highest number of seropositive animals was found in the northeast region (n = 221). In this study, the majority of cattle were sampled from Mymensingh (21%), followed by Chattogram (18.5%), Gazipur (13.7%), Khagrachari (11.5%), Dhaka (11%), Jamalpur (7.6%), Narsingdi (7.4%), Netrakona (5%) and Sherpur (3.7%). Mymensingh district recorded the highest number of seropositive animals (n = 138). Two-thirds (73.4%) of the sampled cattle were female, with females showing higher seropositivity, accounting for 482 of the seropositive cases. The age distribution of the population under study was nearly equal. Cattle older than 1 year exhibited a higher number of seropositive cases (n = 348). Calves, which comprised 48.2% of the total population, had the highest number of seropositive cases (n = 284) among all animals. Among adult cattle, dairy cows showed the highest seropositivity (n = 202). The majority of the sampled cattle were crossbred (75.82%), with a total of 507 seropositive cases in this group (Table 1).
Table 1. Overall status of seroprevalence on different animal-level parameters in cattle (N = 1472) in the study areas of Bangladesh

Herd- and individual-level seroprevalence of anaplasmosis
Regional seroprevalence
In the study, an overall herd-level seroprevalence of 70.6% (n = 89) was observed, while the individual cattle-level seroprevalence was 42.93% (n = 692). Among the 3 regions, the highest herd-level seroprevalence of anaplasmosis was observed in the southeast region (74.4%, n = 29), followed by the central (69.8%, n = 30) and northeast (68.2%, n = 30) regions (Table 2). At the individual cattle level, the southeast region also had the highest seroprevalence (45.93%, n = 203), followed by the central (43.51%, n = 208) and northeast (40.04%, n = 221) regions (Table 2). The results indicated that the cattle populations in the southeast, central and northeast regions of Bangladesh exhibited a high seroprevalence of Anaplasma infections. This widespread presence underscores the importance of monitoring and managing Anaplasma infections to safeguard cattle health in these regions.
Table 2. Seroprevalence of Anaplasma infections among different regions of Bangladesh diagnosed by competitive enzyme-linked immunosorbent assay (cELISA)

CI, confidence interval; n/N, number of positive/number of examined.
District-level seroprevalence
Among the districts, Sherpur district had the highest herd-level seroprevalence (83.3%, n = 5), followed by Gazipur district (81.8%, n = 9), despite the small herd sizes in both cases. Beyond these, Chattogram (80.8%, n = 21) exhibited the highest seroprevalence, followed by Mymensingh (75%, n = 18), Dhaka (73.7%, n = 14), Jamalpur (57.14%, n = 4) and Netrakona (50%, n = 3) districts and Khagrachari (61.5%, n = 8) exhibited the lowest herd-level seroprevalence, respectively (Table 3). Additionally, at individual cattle level, the highest percentages of seropositive cattle were recorded in Khagrachari (48.8%, n = 83), followed by Gazipur (48%, n = 97), Mymensingh (44.5%, n = 138), Chattogram (44.4%, n = 120), Narsingdi (44%, n = 48), Sherpur (40%, n = 22), Dhaka (37.7%, n = 63), Jamalpur (33.9%, n = 38) and Netrakona (30.7%, n = 23) districts, respectively (Table 3).
Table 3. Seroprevalence of Anaplasma infections among different districts diagnosed by competitive enzyme-linked immunosorbent assay (cELISA)

CI, confidence interval; n/N, number of positive/number of examined.
Bovine anaplasmosis risk factors in individual cattle level
At the individual cattle level, regions, districts, sex, age, herd type, herd size and breed were all significantly (P ⩽ 0.05) associated with anaplasmosis (Table 4). The regional- and district-level seroprevalence is described in the previous sections of the study. The southeast region and Khagrachari district had the highest seroprevalence, with both being significantly associated (P = 0.05) with higher rates of infection (Table 4). Additionally, the univariable analysis revealed that female animals had a significantly (P = 0.04) higher prevalence of Anaplasma infections (44.6%, n = 482) compared to male animals (38.3%, n = 150). A higher prevalence of Anaplasma infections was recorded in cattle older than 1 year (45.7%, n = 348), while a lower prevalence was observed in cattle younger than 1 year (40%, n = 284) (Table 4). The present study was carried out on different cattle herd types, i.e. calves, dairy heifers, beef heifers, bulls and dairy cows among the cattle population in the study areas. The results of univariate analysis indicated that Anaplasma infections were significantly (P = 0.0001) more prevalent in dairy cows (52.1%, n = 202) and dairy heifers (43.7%, n = 97), followed by calves (40%, n = 284), bulls (32.5%, n = 41) and beef heifers (30.8%, n = 8), respectively (Table 4). The herd size of animal farms was significantly (P⩽0.05) associated with anaplasmosis, as revealed by univariable logistic regression analysis. The seroprevalence of Anaplasma infection was significantly (P = 0.002) higher in herds with more than 28 animals (81.1%, n = 30) compared to herds with fewer than 4 animals. It was also significantly (P = 0.01) higher in herds with 4–28 animals (72.6%, n = 53). The study also revealed that crossbred animals had a significantly (P = 0.001) higher seroprevalence of Anaplasma infection (45.4%, n = 507) compared to indigenous cattle breeds (35.4%, n = 125) (Table 4).
Table 4. Univariate logistic regression analysis between demographic characteristics and Anaplasma seroprevalence among cattle in different selected dairy farms in Bangladesh

CI, confidence interval.
*Significant at P ⩽ 0.05 level.
Regions and districts with age groups and herd types with sex groups were collinear (Cramer's phi-prime statistic >0.70). Therefore, regions, districts and sex were excluded from the multivariable logistic regression analysis. The odds ratio (OR) of anaplasmosis was significantly (P = 0.01) higher in cattle aged >1 year, with an OR of 1.86 (95% CI 1.4–3.1), compared to cattle aged <1 year (Table 5). For herd type, significantly (P = 0.001) dairy cows had the highest odds of Anaplasma infection (2.25 times, 95% CI 1.48–3.44) followed by dairy heifer (1.68 times, 95% CI 1.02–2.54), compared to bulls. Compared with a herd size of <4, the odds of Anaplasma infection were significantly (P ≤ 0.001) 11.3 (95% CI 7.9–28.2) and 7.45 times (95% CI 4.6–21.56) greater in herd sizes of >28 and 4–28, respectively. Crossbred cattle had significantly (P = 0.001) higher odds of Anaplasma infection, increasing the risk by 2.4 times (95% CI 1.68–3.94) compared to indigenous (Bos indicus) cattle (Table 5).
Table 5. Multivariate logistic regression analysis of important variables (P < 0.05) after collinearity checking associated with Anaplasma seroprevalence among cattle in different selected dairy farms in Bangladesh

CI, confidence interval.
*Significant at P ⩽ 0.05 level.
Discussion
Anaplasma is a tick-borne pathogen that can cause disease in cattle, leading to economic losses in the livestock industry (Rodríguez et al., Reference Rodríguez, Ortiz, Ocampo and Murguía2009). In developing countries like Bangladesh, where there may be limited resources for tick control and veterinary care, bovine anaplasmosis becomes a major problem. This is the first seroprevalence report of Anaplasma infections in the cattle population of Bangladesh. In this study, we estimated seroprevalence of Anaplasma based on the herd and cattle level in the 9 intensive dairy rearing districts of Bangladesh and identified risk factors for Anaplasma infection in cattle.
The study revealed that the seroprevalence of anaplasmosis at regional level varied from 40 to 46% in individual cattle level and 66 to 74% at herd level and the highest seropositivity was found in the southeast region for both cases. In addition, seropositivity was between 32 and 49% at the individual cattle level, while it was 50 and 83% at the herd level in 9 study districts. The study revealed that cattle from Khagrachari, Gazipur, Chattogram and Mymensingh had high seropositivity. This suggests that these animals have had previous or ongoing infections with Anaplasma spp. The seroprevalence of the Anaplasma infection in the present study was consistent with those reported previously from the neighbouring country, India, particularly in southern Rajasthan, India, where the seropositivity was 42.28% in cattle and 48.72% in organized cattle herds, respectively (Sharma et al., Reference Sharma, Bhatnagar, Bhardawaj and Meena2015; Sarangi et al., Reference Sarangi, Rana, Prasad, Ponnanna and Sharma2021). Another seroprevalence study reported 34 and 46% seropositivity for bovine anaplasmosis in India and globally (Paramanandham et al., Reference Paramanandham, Mohankumar, Suresh, Jacob and Roy2019). However, the present study findings conflicted with those reports, where seropositivity of Anaplasma infection was 15.02% in Texas (Hairgrove et al., Reference Hairgrove, Craig, Budke, Rodgers and Gill2014) and 18.5% in Egypt (Parvizi et al., Reference Parvizi, El-Adawy, Melzer, Roesler, Neubauer and Mertens-Scholz2020), respectively.
At the individual cattle level, regions, districts, sex, age and breed were identified as potential risk variables for Anaplasma infection test-positivity, while herd type and herd size were identified as risk variables at the herd level. In the present study, age was determined to be one of the potential risk variables for bovine anaplasmosis. The seroprevalence of Anaplasma infections in cattle aged >1 year had around 2 times higher odds of bovine anaplasmosis compared to that of cattle aged <1 year. The findings were in line with other previous published reports where Anaplasma infections increase significantly with age and have the highest prevalence in adults more than 1 year old (Chowdhury et al., Reference Chowdhury, Hossain, Barua and Islam2006; Kocan et al., Reference Kocan, de la Fuente, Blouin, Coetzee and Ewing2010; Alim et al., Reference Alim, Das, Roy, Masuduzzaman, Sikder, Hassan, Siddiki and Hossain2012; Atif et al., Reference Atif, Khan, Iqbal, Arshad, Ashraf and Ullah2012). This higher seropositivity in adults compared to young animals might be due to a higher chance to pick up the Anaplasma infection as they stay on the farm longer than male cattle. However, these findings conflict with those reports where anaplasmosis was more common in young animals than in adult cattle (Nazar et al., Reference Nazar, Khan, Shah, Rahman, Khan, Ullah, Khan and Shuaib2018; Khan et al., Reference Khan, Sarwar, Ayaz, Ali, Ali, Khan, Khan, Hussain and Ali2019).
In this study, breed was also identified as a potential risk for the occurrence of Anaplasma infections in cattle. Crossbred cattle are 2.4 times more prone to anaplasmosis compared to local/indigenous cattle. The present finding was consistent with the previous reports on anaplasmosis, highlighting the vulnerability of crossbred cattle to Anaplasma infections (Ananda et al., Reference Ananda, D'souza and Puttalakshmamma2009; Siddiki et al., Reference Siddiki, Uddin, Hasan, Hossain, Rahman, Das, Sarker and Hossain2010). In addition, previous reports observed a higher prevalence of infection in exotic breeds and their crosses compared to local breeds of cattle (Chowdhury et al., Reference Chowdhury, Hossain, Barua and Islam2006; Atif et al., Reference Atif, Khan, Iqbal, Arshad, Ashraf and Ullah2012; Farooqi et al., Reference Farooqi, Ijaz, Rashid, Nabi, Islam, Aqib, Hussain, Khan, Rizvi, Mahmood and Mehmood2018; Khan et al., Reference Khan, Sarwar, Ayaz, Ali, Ali, Khan, Khan, Hussain and Ali2019; Shoaib et al., Reference Shoaib, Rashid, Akbar, Sheikh, Farooqi, Khan, Khan and Khan2021). This is attributed to the fact that exotic breeds and their crosses are more susceptible to tick infestation. The lower frequency in indigenous cattle could be due to constant exposure to diseases, leading to the development of immunity against Anaplasma infections. Conversely, the emphasis on the management of crossbred cattle may offer fewer opportunities for pre-exposure to vectors and may result in limited or no immunity, thereby leading to a higher prevalence of the disease (Bock et al., Reference Bock, Vos, Kingston and McLellan1997).
At herd level, herd type also emerged as a potential risk factor for bovine Anaplasma infections where cattle were raised for various purposes, viz., calf, dairy heifer, beef heifer, bull and dairy cow. Dairy cows had more than twice the odds of getting Anaplasma infection compared to calves, and between dairy heifers and beef heifers, dairy heifers were found to be more susceptible to Anaplasma infection while bull had lower odds than dairy cows. Calves are susceptible to anaplasmosis due to transplacental transmission of the disease and may acquire the infection from infected dams through vertical transmission or through exposure to ticks in calving areas or pastures (Radostits et al., Reference Radostits, Blood and Gay2000; Kocan et al., Reference Kocan, de la Fuente, Blouin, Coetzee and Ewing2010; Aubry and Geale, Reference Aubry and Geale2011; Van Loo et al., Reference Van Loo, Pascottini, Hooyberghs, De Bleecker, Ribbens, Opsomer and Pardon2023). The findings were consistent with the reports where A. marginale in dairy animals was higher than bulls and calves (Rajput et al., Reference Rajput, Song-hua, Arijo, Habib and Khalid2005). The greater prevalence of A. marginale in female cattle may be related to lactation in high-producing animals (Kocan et al., Reference Kocan, de la Fuente, Blouin, Coetzee and Ewing2010) and probably because they are kept longer for breeding and milk production, with diets insufficient to meet their high demands. Additionally, the frequent use of contaminated needles to inject medications for milk let-down may contribute to the increased occurrence of tick-borne diseases in dairy animals. Another report suggested that exposure to A. marginale is common in dairy herds (Oliveira et al., Reference Oliveira, Montoya, Romero, Urbina, Soto-Barrientos, Melo, Ramos and Araújo2011).
Size of herds has been reported as a risk factor for anaplasmosis (Okafor et al., Reference Okafor, Collins, Daniel, Coetzee and Whitlock2019; Spare et al., Reference Spare, Hanzlicek, Wootten, Anderson, Thomson, Sanderson, Ganta, Reif and Raghavan2020). In our study, herd sizes of >28 and 4–28 had higher odds compared to herd sizes of <4, and the findings were consistent with the previous reports (Okafor et al., Reference Okafor, Collins, Daniel, Coetzee and Whitlock2019; Spare et al., Reference Spare, Hanzlicek, Wootten, Anderson, Thomson, Sanderson, Ganta, Reif and Raghavan2020). However, another explanation might be the study design in which more cattle were tested in larger herds, which increases the herd-level sensitivity in larger herds. In conclusion, a substantial proportion of cattle and herds tested positive for Anaplasma infection, with herd size and type, age of individuals, sex and breed status significantly associated with the infection in cattle of these selected districts in Bangladesh. The study further suggests that regular health examinations for Anaplasma infection in larger herds, especially targeting older cattle, should be done within the context of Bangladesh.
Data availability statement
The data will be available.
Acknowledgements
The authors acknowledge the support of the Livestock and Dairy Development Project (LDDP), Department of Livestock Services (DLS), Ministry of Fisheries and Livestock, Govt. of Bangladesh and the owners of the commercial cattle farmers. The abstract was presented in the 29th international conference of WAAVP 2023, Chennai, India during 20–24 August 2023. M. M. R. Z. had been awarded travel grant from Dublin Scholarship fund.
Author contributions
M. M. R. Z. and N. A.: review of literature, investigation, resources, methodology, formal analysis, original draft preparation, writing – review and editing. M. A., M. A. H. M., M. M. R. S., M. R. R. R. and M. K. R.: methodology, investigation, formal analysis, writing – review and editing. B. C. R.: co-supervision, resources, methodology, formal analysis, writing – review and editing. M. H. T.: conceptualization, methodology, project administration, supervision, validation, visualization, investigation, resources, writing – review and editing. M. M. R. Z. and N. A. contributed equally to this work.
Financial support
The authors acknowledge the financial support (to principal investigator, M. H. T.; research and innovation subproject; project code: RP-C-03-24) of Livestock and Dairy Development Project (LDDP), jointly funded by the World Bank and Department of Livestock Services (DLS), Ministry of Fisheries and Livestock, Govt. of Bangladesh.
Competing interests
None.
Ethical approval
The study was approved by the Animal Welfare and Experimentation Ethical Committee (AWEEC) of Bangladesh Agricultural University (AWEEC/BAU/2022/12). Both written and oral consent were obtained from the owner/manager of the dairy cattle farm before collecting blood samples and data.