Highlights
-
• Kinematic analysis was used to assess cervical dystonia (CD) movement patterns for botulinum toxin type A injections.
-
• The study compared clinically determined and kinematic-based injections, showing symptom and disability reductions with both.
-
• Kinematic-based injections significantly reduced dystonic movements, suggesting their potential to guide both novice and experienced injectors in treating CD.
Introduction
Botulinum toxin type A (BoNT-A) injection therapy for idiopathic cervical dystonia (CD) is the treatment of choice as it is both effective and well-tolerated.Reference Spiegel, Ostrem and Bledsoe1–Reference Tyślerowicz, Kiedrzyńska, Adamkiewicz, Jost and Sławek3 However, a considerable number of patients experience suboptimal and unsatisfactory outcomes, and approximately 30% of patients discontinue therapy.Reference Tyślerowicz, Kiedrzyńska, Adamkiewicz, Jost and Sławek3,Reference Jinnah, Comella, Perlmutter, Lungu and Hallett4 One of the most common reasons for treatment failure is the incorrect identification of the injection pattern for CD.Reference Tyślerowicz, Kiedrzyńska, Adamkiewicz, Jost and Sławek3,Reference Jinnah, Goodmann and Rosen5 There is no standardized technique to assess and accurately detect dystonic muscle involvement.Reference Sławek and Jost6 Thus, injectors must use clinical experience when selecting the correct muscles and subsequently the doses required.
A recent clinical classification using the “Col-Cap” concept that considers the three dimensions of movement at two levels (caput and collis) may improve muscle selection as the classification is based on muscle anatomy and function.Reference Jost and Tatu7,Reference Pandey, Kreisler and Drużdż8 However, the “Col-Cap” concept relies on subjective assessment to distinguish the respective caput and collis types. Another method to facilitate injection pattern determination (dose and muscle selection) is using objective measures that can provide clinically relevant information to an injector. Our group demonstrated that kinematic (motion-sensor) techniques can measure all the primary degrees of freedom (rotation, lateral flexion and anteroposterior flexion) in simple cases and help separate complex neck movements into these primary motions (e.g., torticaput combined with retrocaput) within an individual.Reference Samotus, Lee and Jog9 In this study, injections based upon kinematic-guided BoNT-A pattern determination significantly reduced motor severity and disability caused by CD.Reference Samotus, Lee and Jog9 Thus, objectively understanding the primary dystonic subtypes, to select muscles that would contribute to these subtypes based on anatomy and function, and the severity of these dystonic movements, to aid with selecting BoNT-A dose per muscle, may improve treatment outcomes. However, the study did not compare the sole use of kinematics versus clinically determined patterns in terms of the efficacy of treatment.
To address this important question, the current study investigated whether BoNT-A injection patterns determined by two dosing approaches – kinematic-based without clinical guidance or a clinical assessment of CD symptoms by a movement disorders neurologist with more than 30 years of experience – result in similar efficacy and tolerability outcomes. The injection is performed by a separate expert injector blinded to the method of assessment. In this prospective, independent armed study, a total of 22 BoNT-A naïve CD patients were randomized to receive 3 serial BoNT-A injections determined either clinically or kinematically and administered by the blinded injector.
Methods
Study design and participants
This open-label, single-site, non-inferiority study was conducted at the London Movement Disorders Centre between October 2016 and March 2020 with approval by the Western University Health Sciences Research Ethics Board (REB#105515) and was registered on the ClinicalTrials.gov registry (NCT02662530). Participants provided written informed consent prior to study initiation. A convenience sampling of a total of 22 CD participants was alternately assigned into two groups – a clinic-based (“cb”) or kinematic-based (“kb”) assessment group – based on their recruitment sequence (1:1). All participants were blinded to which group they were assigned to. Eleven CD participants were treated using clinic-based (“cb”) injection patterns, determined by the expert injector (Dr Mandar Jog [MJ]), and 11 participants were treated using injection patterns solely determined using kinematic analysis without any clinical guidance (“kb”). All participants were injected with BoNT-A (onabotulinumtoxinA; Botox®, 1.0 mL of saline per 100-unit vial) under electromyographic (EMG) guidance to localize the targeted muscle (Clavis®, Natus Medical Incorporated, Pleasanton, California, USA) with a 1” long × 30G injectable EMG needle by an expert injector (KS) over three serial injections at weeks 0, 12 and 24. The injector (KS), who does not have CD dosing experience but is well-versed in cervical anatomy and injects these muscles in their physiatry clinic, was blinded to the treatment group of each participant (“cb” or “kb”) and to all clinical and kinematic measures. The assessor was blinded to the treatment cohort to which the participants were allocated. The peak effect of BoNT-A was measured at weeks 6, 18 and 30. Clinical scale scores and kinematic analysis of CD symptoms were collected at all six study visits. See Figure 1 for the CONSORT diagram outlining the enrollment and study design.
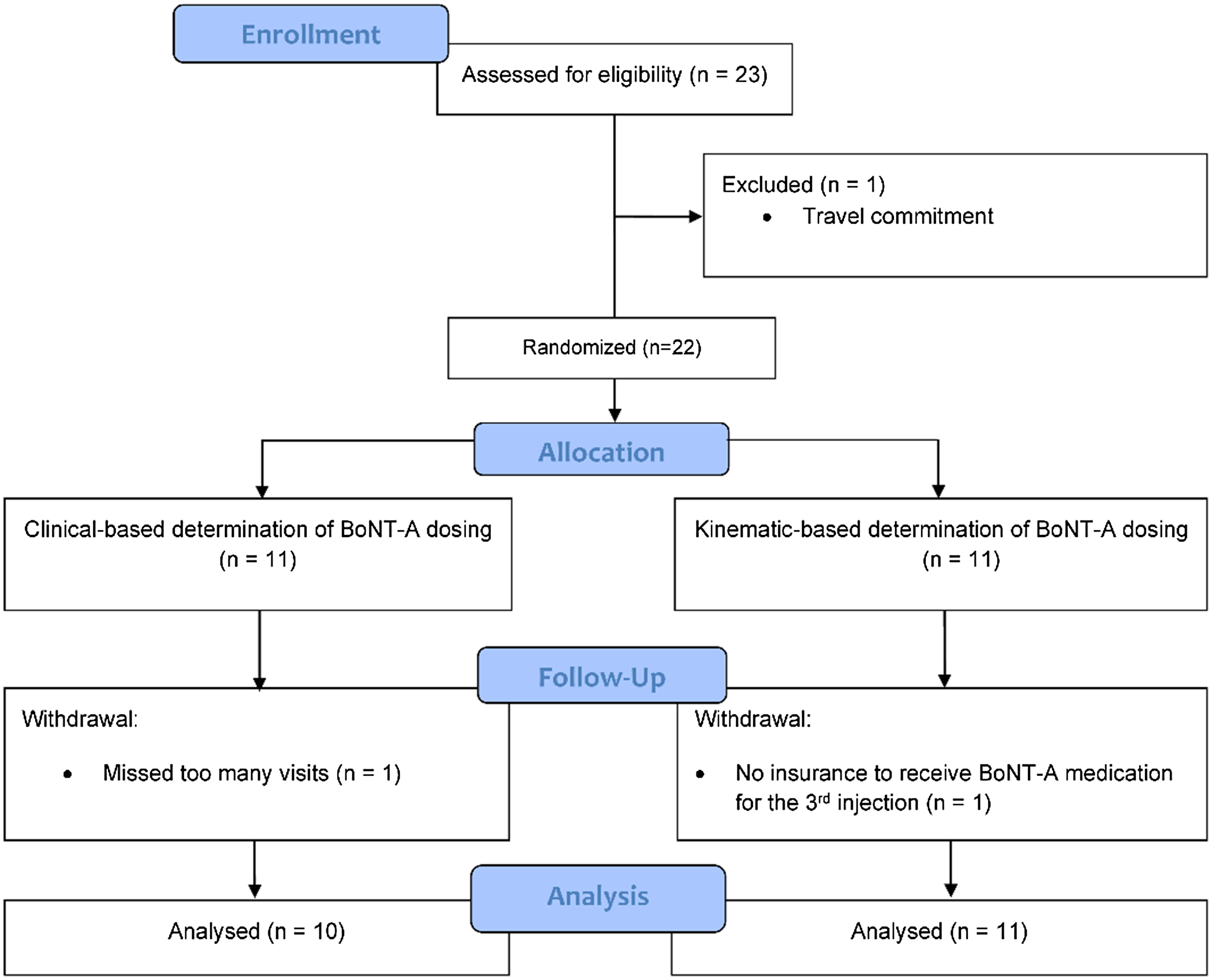
Figure 1. The study design, number of participants in the study and the participants included for analysis are illustrated in a Consolidated Standards of Reporting Trials (CONSORT) diagram.
Inclusion criteria were 18–80-year-old participants with idiopathic CD, naïve to BoNT-A or at least 6 months since prior injection (for a full washout period), able to attend all study visits and able to provide written consent. Exclusion criteria were contradictions to BoNT-A (Botox®, AbbVie, Illinois, USA) as per the monograph, pregnancy, nursing or planning a pregnancy, cervical contractures limiting range of motion, known or suspected traumatic causes of CD, thalamotomy or peripheral operation, motor/nerve or other neuromuscular junction diseases and prior myotomy or denervation surgery involving neck/shoulder region.
Outcome measures
At each study visit, the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS), kinematic assessment and treatment-related side effects were conducted. TWSTRS scores were collected to evaluate clinical motor severity (part I), disability (part II) and pain (part III). For kinematic CD assessment, two motion sensors, a goniometer and a torsiometer (W180 and Z180, Biometrics Ltd., Newport, UK), were utilized to capture head movements by attaching both sensors to the back of each participant’s head at the inion and spinal segments C5–C6 using Velcro straps (Figure 2). These sensors allowed simultaneous measurements of tonic deviation (degree of deviation from neutral head position [>5°] and presence of dynamic dystonic movements (measured in root mean square [RMS] degrees [≥0.1°]) in three planes of motion: left/right lateral, forward/backward (vertical) and left/right rotational deviations. Participants were instructed not to resist dystonic movements (at rest) while eyes were open and closed (recording was done after 1 minute of eyes closed) over four trials (10 seconds/trial). A neutral head position was performed by the assessor holding the participants’ heads straight (nose/larynx medial and in line with the chest/torso and chin parallel to the floor). Raw data was captured using DataLITE sensor acquisition software (PC software version 8.7, Biometrics Ltd., Newport, UK) and analyzed using a custom algorithm written in MATLAB® (R2014b, MathWorks, Natick, MA, USA) software.
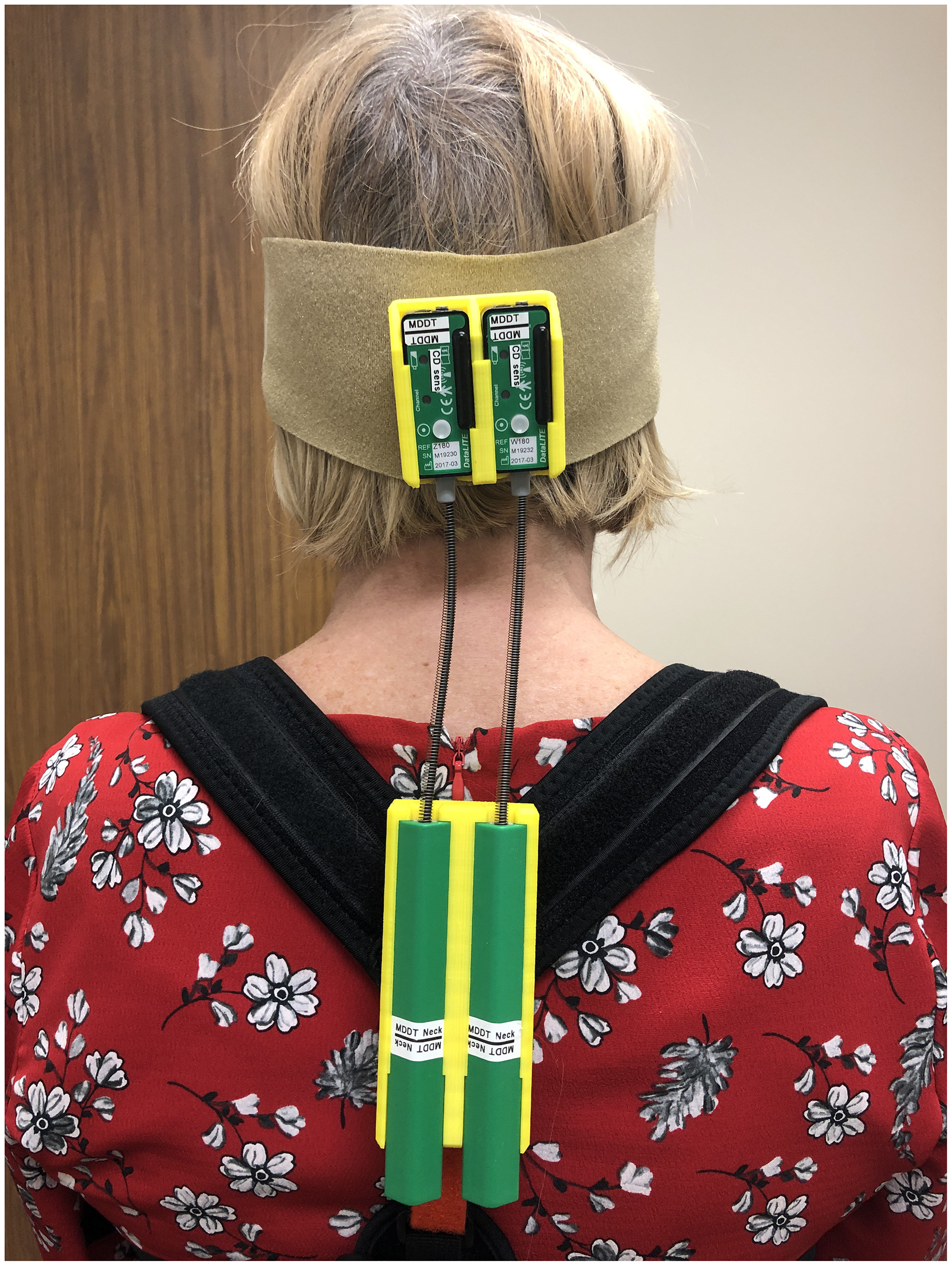
Figure 2. Depiction of the placement of the torsiometer and goniometer sensors for kinematic cervical dystonia assessment.
BoNT-A dosing
Initial injection patterns for the clinic-based group (“cb”) were determined solely by the movement disorder neurologist (MJ) with more than 30 years of BoNT-A dosing experience for CD. The clinical assessment involved visual and muscle palpation to distinguish which muscles were primarily involved and contributed strongly to the most severe dystonic movements. Optimization of injection patterns for the “cb” group was conducted by the same movement disorder neurologist and was based on a clinical assessment of the participant at the follow-up and re-injection time-points and using participant feedback.
Initial injection patterns for the kinematic-based group (“kb”) were determined solely using kinematic analysis results without any clinical guidance or interpretation from the movement disorder neurologist. The methodology for determining kinematic-based injection patterns was previously reported.Reference Samotus, Lee and Jog9 First, the primary (most severe) dystonic tonic posturing (head deviation > ±5° from neutral head positioning per direction as <5° was deemed not clinically significantReference Samotus, Lee and Jog9 ) was identified. Dependent on the extent and direction of the primary CD symptom, muscles associated with each plane of motion were targeted for treatment.Reference Samotus, Lee and Jog9 Selected BoNT-A dosages per muscle corresponding to the degree/severity of movement (static or dynamic) per plane of motion were based on the results from our center’s pilot study.Reference Samotus, Lee and Jog9 Dose per muscle was not tailored to participants’ age, gender, muscle mass or EMG activity.
Muscle selection and dose per muscle were dependent on the severity of the primary tonic dystonia reported by the kinematic data. For example, 20 U each in the ipsilateral splenius capitis (SC) and the contralateral sternocleidomastoid (SCM) muscles was allocated for a torticaput, 10° from neutral, whereas 35 U each in the ipsilateral SC and contralateral SCM muscles was allocated for 35° torticollis. If the participant had no tonic head deviation (within ±5° from a neutral position in all planes of motion), then the injection pattern was solely dependent on the primary dynamic (tremulous) movements. For example, a participant with primarily rotational tremor between 0.3 and 1.0 RMS degrees was allocated an initial pattern of 20 U each in the bilateral SC and SCM muscles. For participants with both tonic and dynamic movements, some muscles may be repeatedly selected, and thus, a 5 U dose increase for overlapping muscle(s) was utilized. For example, in a patient with 35° tonic torticollis and 0.4° RMS degree of dynamic rotational movements, the injection pattern would be 35 U in the ipsilateral SC, 25 U in contralateral SCM and 20 U in the ipsilateral SCM totaling 80 U. The presence of either mild/moderate or severe shoulder elevation was allocated 20 U or 30 U, respectively, each in the ipsilateral levator scapulae and ipsilateral descending trapezius muscles. Optimization of kinematic-based injection patterns was based on the changes in tonic deviations and/or dynamic movements and using participant feedback at the 6-week follow-up compared to the injection visits. Any treatment-related side effects such as the presence of dysphagia or muscle weakness resulted in a dose reduction at the next injection, in the muscle(s) contributing to the side effect.
Statistical analysis
TWSTRS sub-scores for parts I–III were reported as median and mean ± standard deviation of all participants per treatment group for each visit. The presence of tonic head deviation (>5°) and dynamic (tremor/dystonic) movements (RMS degrees > 0.1) in all affected plane(s) of motion were averaged over the four trials per participant at each visit. The baseline severity of the tonic deviation and dynamic movements for all degrees of freedom that were affected for each participant were summarized in Table 1. The medians per treatment group for each visit were reported. All statistical tests were performed using IBM® SPSS® version 20. Due to the small sample sizes and non-normal distributed data in both treatment groups, nonparametric statistical tests were performed. Dose per muscle, total dose, number of muscles per treatment cycle and baseline clinical and kinematic scores at each time-point were compared between “cb” and “kb” treatment groups using a Kruskal–Wallis H test. A Friedman test with Bonferroni correction for multiple comparisons (α = 0.05) and Kendall’s W was reported to compare TWSTRS total score and sub-scores and kinematic analysis (tonic deviation and dynamic movements) results from week 0 to post-treatment time-points (weeks 6, 12, 18, 24 and 30), peak effect time-points (weeks 6, 18 and 30) and re-injection time-points (weeks 0, 12 and 24) per treatment group. Descriptive statistics (percentages) were used to summarize changes in BoNT-A dosing between treatment groups.
Table 1. Summary of baseline cervical dystonia participant demographics
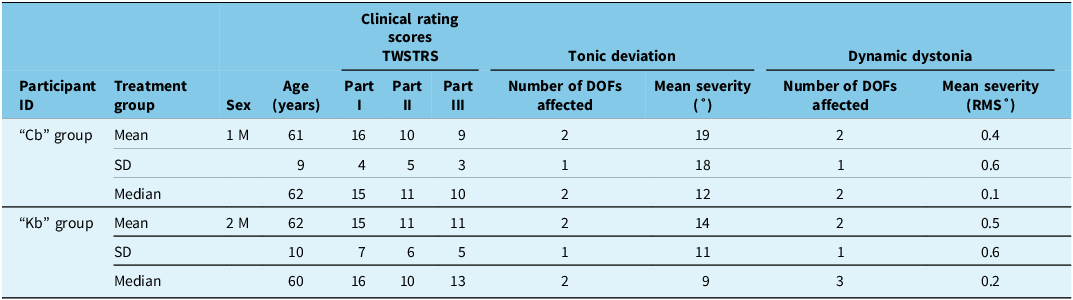
Note: cb = clinic-based treatment group; DOFs = degree of freedoms; F = female; kb = kinematic-based treatment group; M = male; part I = torticollis severity; part II = disability scale; part III = pain; RMS° = root mean square degrees; TWSTRS = Toronto Western Spasmodic Torticollis Rating Scale.
Results
Participant demographics and kinematic phenotypes of CD
Baseline participant demographics, TWSTRS sub-scores and kinematic analysis (mean degree of tonic deviation (sustained abnormal head posturing) and mean tremulous/dystonic dynamic movements in all affected degrees of freedom per participant) are fully detailed in Supplementary Table 1, and the summary of the baseline characteristics are in Table 1. The demographic characteristics between the treatment groups were comparable as there were no significant differences in TWSTRS sub-scores during the treatment period. Tonic deviation in at least two planes of motion was observed in four (40%) “cb” and seven (64%) “kb” participants. Eight (80%) “cb” and 11 (100%) “kb” participants had dynamic movements in at least 1 plane of motion. Three (30%) “cb” and seven (64%) “kb” participants had dynamic movements in three planes of motion.
Clinical versus kinematically based BoNT-A dosing
Total BoNT-A dose and number of muscles injected per treatment cycle were not significantly different between “cb” and “kb” treatment groups (X 2 (1) = 0.040, p = 0.842) (Table 2). However, a median number of muscles injected were higher in the “kb” group. Injection patterns were changed for 7 (70%) and 4 (40%) “cb” participants at the 2nd and 3rd injection cycles, respectively, whereas 10 (91%) “kb” participants required pattern changes at the 3rd injection cycle (Table 2). One “cb” and four “kb” participants required muscle changes at the 3rd injection. A mean of 59% of “kb” participants required an increase in dose per muscle compared to a mean of 45% of “cb” participants at the 2nd and 3rd injection cycles.
Table 2. Mean dose per muscle and mean ± standard deviation and median dose for all muscles, total dose and the number of muscles injected over the three treatment cycles per treatment group
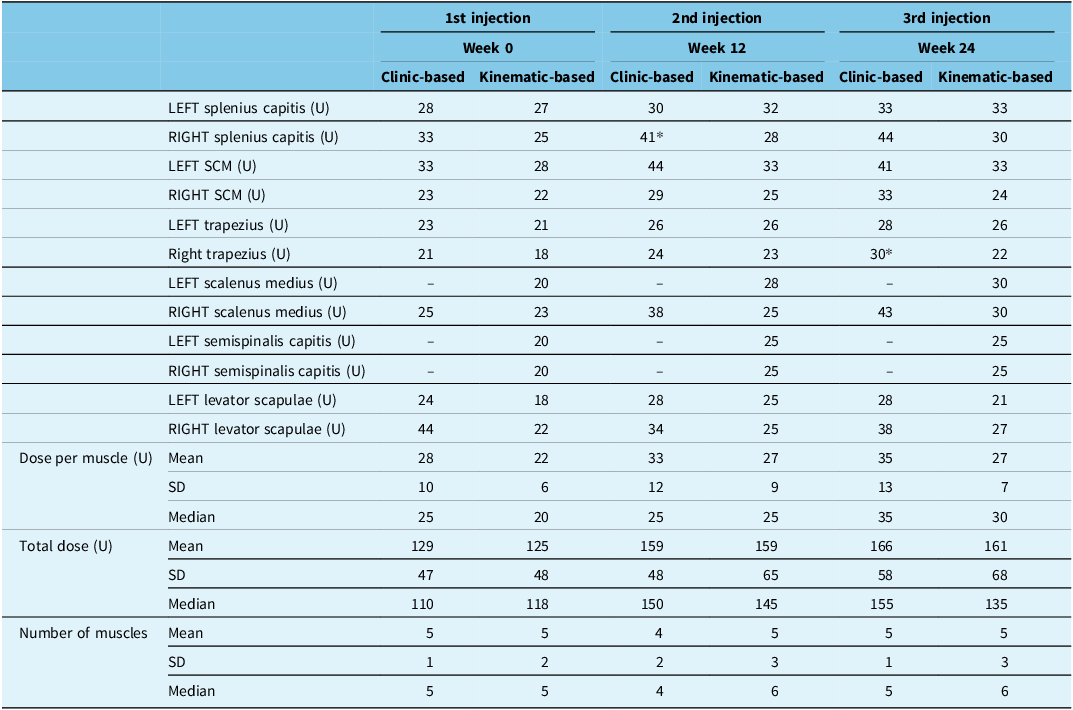
Asterisks represent a statistically significant (p < 0.05) difference in dose compared to the other treatment group in the treatment cycle. SCM = sternocleidomastoid; SD = standard deviation; U = BoNT-A (Botox®) units.
Kinematic outcomes
Median tonic deviation did not significantly improve in either “cb” (X 2 (5) = 3.971, p = 0.554, W = 0.3) or “kb” (X 2 (5) = 5.820, p = 0.324, W = 0.3) treatment groups, although the median tonic deviation was lower in the “kb” group (Figure 3a). A mean of 42% of “cb” participants returned to neutral head positioning after the 1st and 3rd injection cycles (Figure 3b). A mean of 47% of “kb” participants returned to within neutral head positioning (± 5° from neutral in any plane of motion), which was maintained after all injection cycles.

Figure 3. Median kinematic changes in tonic deviations (neck posturing) from neutral (a), the mean percentage of participants who returned to neutral (alleviation of tonic deviation) (b) and median dynamic movements (c) per treatment group over the study.
In the “cb” group, median dynamic movements (RMS degrees) did not significantly change over the treatment course (X 2 (5) = 5.531, p = 0.355, W = 0.4) (Figure 3c). In the “kb” group, median dynamic severity significantly reduced at week 12 (median = 0.1; X 2 (5) = 2.000, p = 0.023, W = 0.4) and was maintained up to week 30 (median = 0.1; X 2 (5) = 2.889, p = 0.001, W = 0.4) compared to week 0 (median = 0.2).
Clinical outcomes
For the “cb” group, the mean total TWSTRS score was significantly reduced to 25.2 ± 7.1 (median = 24.0; X 2 (5) = 1.833, p = 0.038, W = 0.5) and 17.2 ± 11.0 (median = 18.0; X 2 (5) = 3.444, p < 0.001, W = 0.5) at weeks 18 and 30, respectively, compared to week 0 (mean = 34.7 ± 9.7; median = 37.3) (Figure 4a). Mean dystonia severity (TWSTRS part I) was significantly reduced to 12.0 ± 2.8 (median = 13.0; X 2 (5) = 2.0, p = 0.023, W = 0.6) at week 6 and further reduced to 10.8 ± 2.8 (median = 11.0; X 2 (5) = 3.611, p < 0.001, W = 0.6) at weeks 18–30 compared to 16.1 ± 4.3 (median = 15.0) at week 0 (Figure 4b). Mean disability caused by CD (part II) was significantly reduced to 6.3 ± 5.2 (median = 5.0; X 2 (5) = 1.778, p = 0.044, W = 0.5) and 3.1 ± 2.5 (median = 3.0; X 2 (5) = 3.167, p < 0.001, W = 0.5) at weeks 24 and 30, respectively, compared to 9.7 ± 5.3 (median = 10.0) at week 0 (Figure 4c).
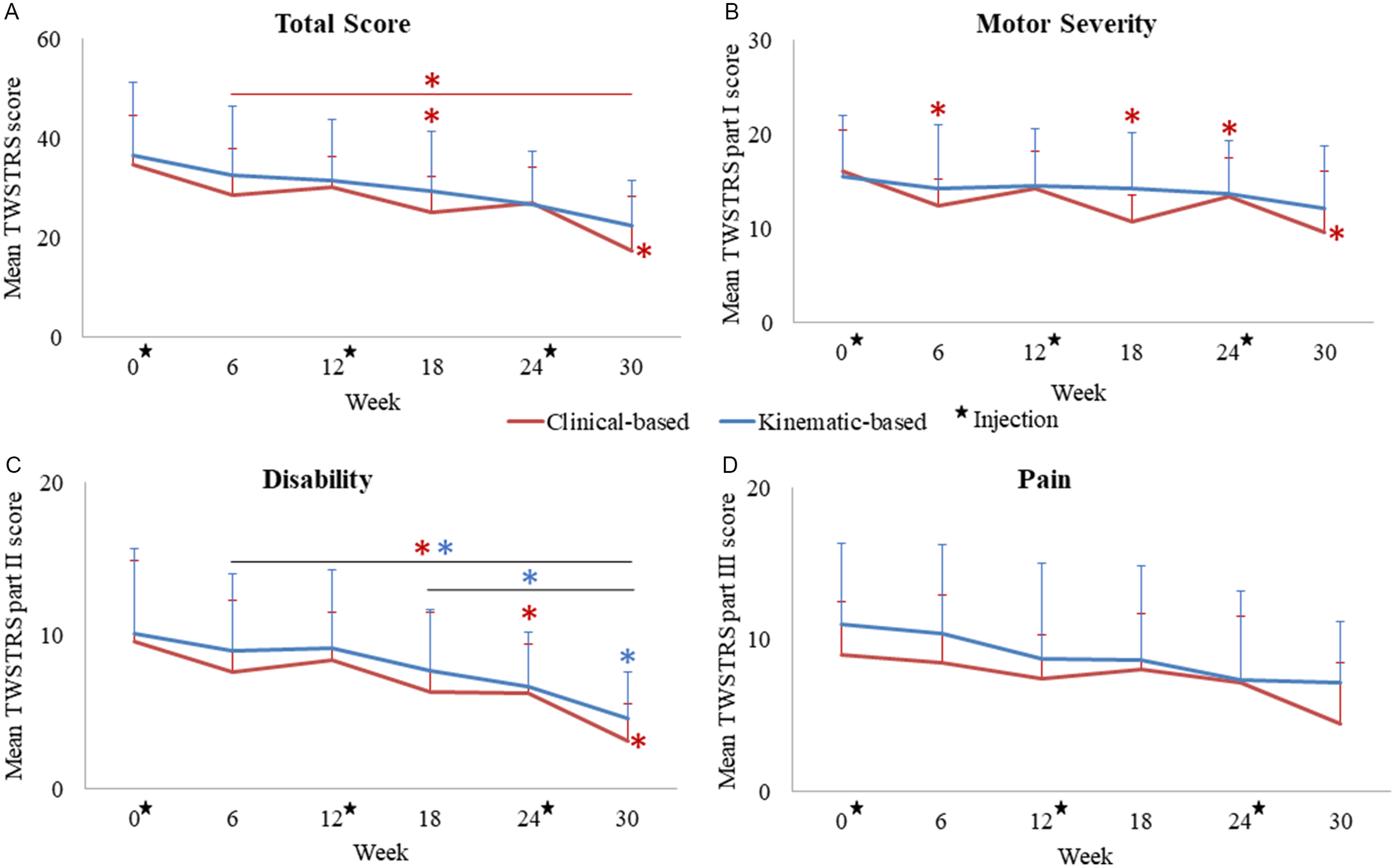
Figure 4. Mean changes in TWSTRS total score (a), motor severity (part I) (b), disability caused by CD (part II) (c) and pain (part III) (d) in both treatment groups over the three injection cycles. Stars represent injection visits, colored asterisks (*) represent statistically significant changes compared to week 0 or between the two time-points denoted by a horizontal line per treatment group. Error bars represent standard deviation.
For the “kb” group, the mean disability score significantly decreased to 4.6 ± 3.0 (median = 5.0; X 2 (5) = 2.312, p = 0.013, W = 0.4) at week 30 compared to 10.2 ± 5.5 (median = 9.0) at week 0. TWSTRS total score was nonsignificantly reduced to 26.3 ± 14.7 (median = 26.0) at week 30 from 37.6 ± 15.4 (median = 39) at week 0 (Figure 4a). TWSTRS motor severity sub-score for the “kb” group did not significantly change (Figure 4b), and there were no significant changes in pain severity in both groups (Figure 4d).
Side effects
In the “cb” group, one participant had treatment-related muscle weakness and pain in the left SC area following the 2nd injection, and one participant experienced muscle weakness resulting in a “pulling” forward of the head (injected 37.5 U in right SCM) following the 3rd injection.
In the “kb” group, a participant had treatment-related muscle weakness, resulting in “pulling” forward of the head (injected 30 U and 15 U in left and right SCMs, respectively) following the 1st injection, and three participants had moderate dysphagia following the 2nd injection. A dose reduction (n = 1) in the SCM (from 50 U to 40 U), double concentration to minimize spread of BoNT-A (n = 1) and a study withdrawal following the 3rd injection due to additional adverse effects unrelated to BoNT-A of elevated blood pressure and diffuse pain throughout body (n = 1) were observed.
Discussion
Identifying muscles that contribute to dystonic neck movements and selecting appropriate BoNT-A dosages to treat CD are challenging for injectors. Although guidelines have been published to aid clinicians to initiate therapy and manage outcomes,Reference Reichel, Stenner and Jahn10,Reference Albanese, Abbruzzese and Dressler11 there is no validated approach for determining the pattern of deviation. This deficiency is reflected in subsequently mis-selecting which muscles and the ideal dose per muscle,Reference Jost, Drużdż and Pandey12 which results in poor outcomes, side effects and dropout of patients. There is a significant lack of such expert injectors, and training programs require prolonged fellowship training to become competent, resulting in a shortage of experienced injectors and poor injector access for patients. In this study, we compared the treatment outcomes of participants injected by a blinded injector using two different dosing approaches: kinematic-based (“kb”; without clinical guidance) and clinic-based (“cb”; using clinical guidance) injection patterns. The goal of the study was to confirm that assessment using “kb” was at least as good as “cb” performed by a highly experienced injector. The method of “kb” assessment could potentially be used as an adjunct to clinical assessment in the hands of novice injectors, for complex cases for experienced injectors and even as a teaching tool.
A significant reduction in tremulous dynamic movements and a sustained alleviation of kinematic tonic neck posturing were observed in the “kb” group over the three treatments. Participants treated using “cb” injection patterns experienced nonsignificant reductions in tremor/dynamic severity; however, reductions in kinematic tonic posturing were not sustained after the 1st injection (week 12 and onward). Significant changes in CD symptoms observed in the “kb” group may be contributed by kinematics providing the granularity required to tailor dosages of BoNT-A to appropriate muscles. Separating the superimposed, multiaxial dynamic spasmodic movements from tonic deviations is challenging for injectors for selecting appropriate injection patterns. The severity of the primary dystonia that may include both tonic and dynamic cervical movements was used to identify the ideal starting dose and select muscles contributing to the primary dystonia. Starting dosages relating to the severity of tonic and dynamic dystonic movements per degree of freedom were first identified in our pilot study.Reference Samotus, Lee and Jog9
TWSTRS disability sub-score was significantly reduced in both treatment groups, while the “cb” group also had significant reductions in the motor severity sub-score. The lack of significant improvement in the motor severity score, which assesses static dystonic movements (e.g. rotation, laterocollis, anterocollis/retrocollis), observed in the “kb” group may be due to a higher number of “kb” participants having tremulous movements. The TWSTRS severity sub-score only rates tonic deviations/maximum excursion in each plane of motion, but it does not allow the injector to determine the contribution of the two/multiple different components. It simply sums them up and assumes that all of it must be injected. The TWSTRS does not consider the severity of dynamic movements, which may explain the lack of significance observed in the “kb” group. It is the deficiency of the TWSTRS and not that of the kinematic analysis that may demonstrate the benefit of using an objective measure rather than a subjective scale. However, the changes in TWSTRS total score (∼-10 TWSTRS points indicating minimal clinical improvement) observed in both study treatment groups were still comparable to previously published efficacy studies.Reference Tyślerowicz, Kiedrzyńska, Adamkiewicz, Jost and Sławek3,Reference Jankovic, Adler and Charles13–Reference Trosch, Espay and Truong15 Thus, a standardized approach using kinematics to assess and dose patients may trend toward producing similar clinical outcomes compared to the clinical guidance approach.
Dosages per muscle and total dose used at the 2nd and 3rd injections were comparable to a study that utilized the Col-Cap classification guidelines.Reference Pandey, Kreisler and Drużdż8 Although the total dose and the number of muscles per treatment were similar between groups, the median number of muscles was higher in the “kb” group. The “kb” group also required a greater number of doses and muscle changes at the 2nd and 3rd injection cycles. Furthermore, higher dosages in the (right) SC and (right) trapezius in the “cb” group were observed and may be due to the lower number of muscles injected per cycle. This suggests that optimizing injection patterns using kinematics may provide granularity to the primary dystonic symptoms (in both tonic and dynamic movements) refining muscle selection.
The most common treatment side effects were muscle weakness and dysphagia, as seen in previous treatment studies.Reference Jankovic, Adler and Charles13,Reference Trosch, Espay and Truong15,Reference Bledsoe and Comella16 A similar number of treatment-related muscle weakness were observed in participants in both groups. However, the “kb” group had a larger number of patients affected by dysphagia, which resolved in two participants receiving a dose reduction in the SCM muscles and by minimizing the spread of toxin by a double concentration reconstitution of BoNT-A. Dysphagia is typically related to SCM injections, and all 11 “kb” participants had a tremulous component. It is now recognized that torticaput tremor (no-no tremor) is commonly present in the obliquus capitis inferior (OCI) muscles and that targeting this deep muscle instead of the SCM may improve this type of tremor and avoid dysphagia side effects when the SCM is injected.Reference Pandey, Kreisler and Drużdż8,Reference Bessemer and Jog17 In this cohort of patients, the majority of patients did not have this phenotype on clinical examination. Thus, this may explain the higher incidence of dysphagia in the “kb” group, and it is suggested to target the OCI for caput rotational tremor. The OCI muscle was not in the muscle groups included in the “cb” or “kb” dosing algorithm.
This study demonstrated the utility of kinematic-based BoNT-A injection patterns for effective and tolerable CD symptom management. Kinematics can provide benefit to the clinical expertise and training of a movement disorders neurologist. Differentiating tonic and dynamic movements of CD, this enables personalization of injections, development of an “ideal” dose per muscle and the technology to advance assessment and outcomes for CD therapy. Kinematic technology may be advantageous for novice injectors to enhance their clinical practice or for high-volume injectors in community-based practices. In the community setting, patients who are in need of BoNT-A therapy must wait lengthy times before seeing a movement disorder specialist. Access to this kinematic technology for both novice injectors may reduce these wait times, thereby improving patient care. This is a promising approach that may improve treatment outcomes for complex dystonic cases and those already receiving BoNT-A therapy; however, future studies are needed to confirm this. Although the kinematic assessments can be performed in under 10 minutes by a technician with straightforward training instructions for putting sensors on the patient and running the software, the devices are relatively expensive and can cost up to CAD 10,000 and are currently only available for research studies. Another method to identify muscles contributing to CD symptoms is using EMG coherence analysis, although using this approach solely has not shown to be reliable for CD.Reference Nijmeijer, de Bruijn and Forbes18 However, the use of EMG (Yale protocol) has shown promise for screening upper limb muscles for essential tremor BoNT-A therapy.Reference Mittal, Jog, Lee and Jabbari19 Thus, kinematics simplifies the assessment of complex CD subtypes that involve both tonic and dynamic components in all three planes of motion and standardizes injection pattern determination.
The value of the outcomes reported in this study is limited by the study’s smaller cohort compared to other toxin studies using objective or standardized dosingReference Wu, Xue and Chang20,Reference Nijmeijer, Koelman and Standaar21 and the lack of OCI muscle targeting. Future studies including a larger cohort, single- or multi-site crossover and flexible treatment interval trial design comparing the efficacy and safety of these two dosing approaches should be considered. In addition, the inclusion of multiple assessors should be considered for subsequent studies to enhance the robustness of clinical comparisons while considering the potential variability in dosing patterns.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/cjn.2025.10109.
Data availability statement
Data is contained within the article.
Acknowledgments
The authors would like to acknowledge the contribution of the participants and the researchers who assisted in this project at the National Parkinson Foundation Centre of Excellence, London Movement Disorders Centre located in London, ON, Canada.
Author contributions
Conceptualization, M.J.; methodology, M.J. and O.S.; software, M.J. and O.S.; formal analysis, O.S.; investigation, K.S., M.J. and O.S.; resources, M.J.; data curation, O.S.; writing – original draft preparation, O.S.; writing – review & editing, K.S., M.J. and O.S.; visualization, M.J.; supervision, M.J.; project administration, K.S., M.J. and O.S.; funding acquisition, M.J.
Funding statement
This study was partially funded by a research grant from Allergan Pharmaceuticals during the conduct of this study.
Competing interests
Jog is a scientific advisor and receives research financial support from the following companies: AbbVie, Allergan Inc., Boston Scientific, Ipsen, MDDT Inc., Medtronic, Merz Pharma, Novartis and Teva Pharmaceuticals. In addition, Jog has two patents PCT/CA2013/000804 and PCT/CA2014/050893 pending that are assigned to MDDT Inc. Jog is a shareholder of MDDT Inc. MDDT Inc. has contract agreements with both Merz Pharma and Allergan on the commercial applications of TremorTek™ and Hinge Diagnostics™. Sequeira reports no conflict of interests. Samotus collected and analyzed the study data and co-wrote the manuscript as a researcher at the London Movement Disorders Centre – London Health Sciences Centre prior to joining Ipsen Biopharmaceuticals Canada Inc. Samotus is currently an Ipsen Biopharmaceuticals Canada employee.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the Western University Health Sciences Research Ethics Board (protocol# 105515 on February 10, 2016).








