Background
The rise in nutrition-related illness highlights the need for targeted health promotion and interventions across the lifespan and for future generations( Reference Lopez, Mathers and Ezzati 1 ). Traditionally the focus of such interventions was on prevention of chronic disease and premature death. However, there is now a large body of evidence demonstrating that cognitive decline accompanies certain metabolic health conditions such as type 2 diabetes, metabolic syndrome and obesity and that modifiable lifestyle factors including diet may contribute significantly to the risk of cognitive decline, including dementia( Reference Kanoski and Davidson 2 ). Consequently, there has been an increasing interest in the effects of nutrition on cognitive performance and more specifically how cognitive performance can be optimised using nutritional interventions. When looking across the lifespan, broadly speaking nutritional interventions offer opportunity to (i) optimise cognitive development during infancy and childhood, (ii) ensure the highest levels of cognitive function during adulthood and (iii) prevent cognitive decline in older age (see Fig. 1).
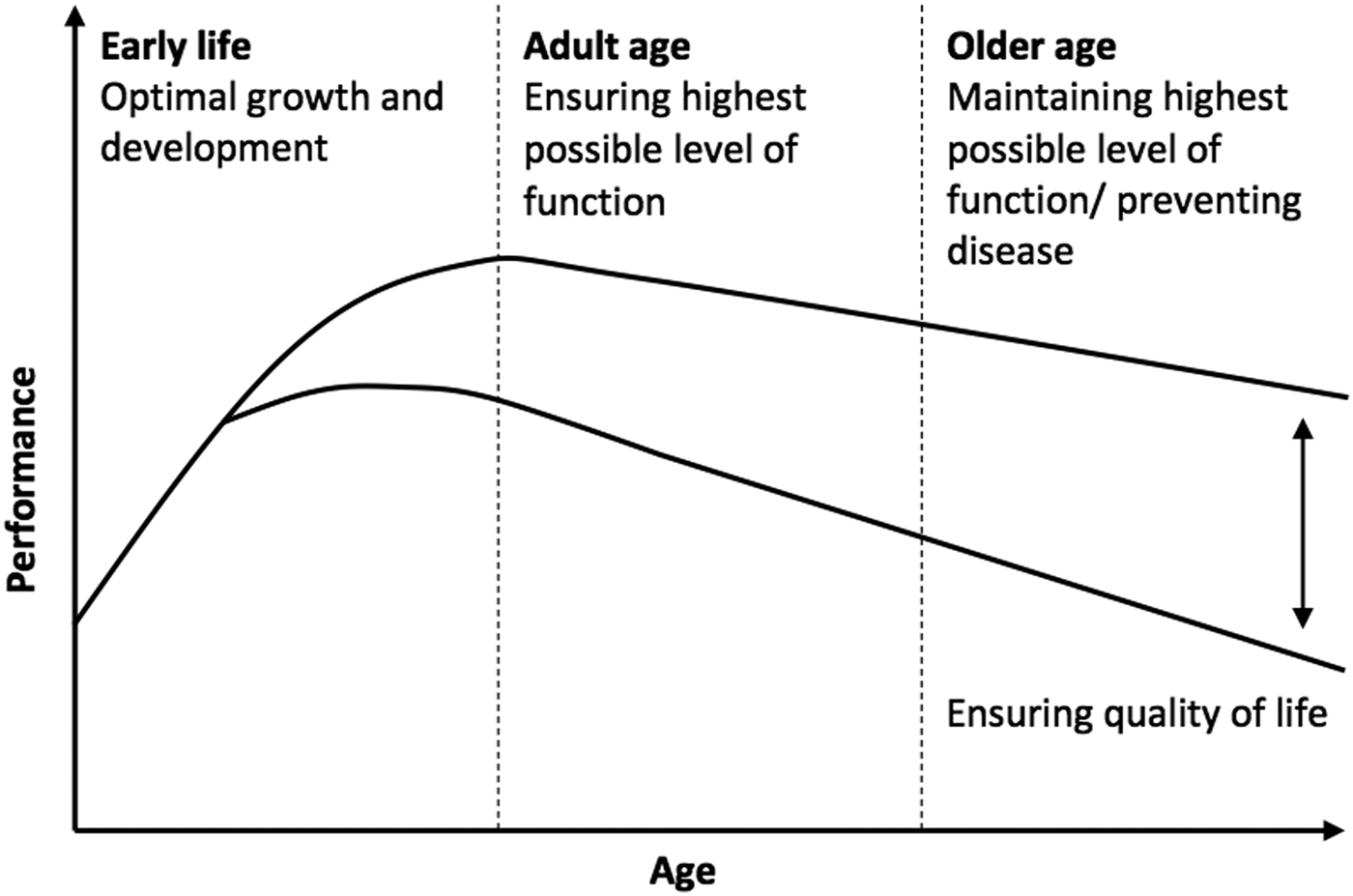
Fig. 1. Nutrition and cognition: potential for optimising cognitive performance across the lifespan.
The macronutrient glucose is perhaps most thoroughly researched in terms of its effects on cognition. Here the impact of diet-based glycaemic response and glucose regulation on cognitive processes across the lifespan will be reviewed. Before considering the relationship of glucose, glycaemic response and cognitive processes, some features of glucose metabolism important for the understanding of its role in cognition will be discussed.
Glucose: the major source of energy for the brain
All processes of cells (including nerve cells) require energy. In human subjects and most animals, ATP works as the main carrier of chemical energy. The human body uses three types of molecules to yield the necessary energy to drive ATP synthesis: fats, proteins and carbohydrates. Aerobic carbohydrate metabolism is the main source of energy available for brain tissue( Reference McIlwain and Bachelard 3 ). Compared with other organs, the brain possesses paradoxically limited stores of glycogen, which without replenishment are exhausted in up to 10 min. In nervous tissue, glycogen is stored in astrocytes. Astrocytes participate significantly in brain glucose uptake and metabolism and due to their location and metabolic versatility( Reference Lehninger, Nelson and Cox 4 ). The entry of glucose into the brain is mediated by the family of GLUT, which are adapted to the metabolic needs of the tissue in which it is found. The primary GLUT isoforms in the brain are GLUT1 and GLUT3 but others have been detected in different brain regions, at a lower level of expression( Reference Maher, Vannucci and Simpson 5 ).
The immense expenditure of energy by the brain relative to its weight and volume is thought to be due to the need to maintain ionic gradients across the neuronal membrane( Reference Ames 6 ). In addition, there is no break from the brain's energy demand as the rate of brain metabolism is relatively steady day and night, and may even increase slightly during the dreaming phases of sleep( Reference Maquet 7 ). Thus the energy requirements of brain tissue are exceptionally constant( Reference Clarke and Sokoloff 8 ) and glucose deprivation can severely disrupt neuronal activity, producing electroencephalogram patterns characteristic of lowered cognitive functioning( Reference Holmes, Hayford and Gonzalez 9 ). Indeed, when blood glucose drops below 4 mm/l (72 mg/dl; hypoglycaemic condition), it can cause discomfort, confusion, coma, convulsions, or even death in extreme conditions( Reference Nelson, Cox and Lehninger 10 ). Conversely, persistent blood glucose concentrations above the normal range (hyperglycaemic condition) can also have damaging physiological effects. Because glucose exerts osmotic pressure in the extracellular fluid, extremely high blood glucose concentrations can cause cellular dehydration, loss of glucose in the urine, which can affect kidney function and deplete the body's supply of fluids and electrolytes( Reference Guyton and Hall 11 ).
Glucose brain metabolism: changes across the lifespan
The rate of glucose brain metabolism changes across the lifespan. Initially, there is a rise in the rate of glucose utilisation from birth until about age 4 years, at which time the child's cerebral cortex uses more than double the amount of glucose compared with adults. This high rate of glucose utilisation is maintained from age 4 to 10( Reference Moeller, Ishikawa and Dhawan 12 ). Childhood is a time of intense learning and therefore coincides with the most metabolically expensive period( Reference Haymond 13 ). The high energy demand of a child's brain requires the use of the majority of hepatically generated plasma glucose( Reference Haymond and Sunehag 14 ). In addition, glucose supply needs to be particularly stable as impairments are thought to occur at higher plasma glucose level (4·2 mm/l)( Reference Jones, Borg and Boulware 15 ). After this period, there is a gradual decline in glucose metabolic rate, reaching adult values by age 16–18 years( Reference Chugani 16 ). This is followed by a plateau phase until middle age after when a significant age-related decline in cerebral glucose metabolism can be observed( Reference Moeller, Ishikawa and Dhawan 12 ). This age-specific metabolic pattern of glucose consumption has not been observed in other species and it has been argued that this could be a driver or indeed a consequence of human cognition( Reference Caravas and Wildman 17 ).
Most children and young adults maintain circulating glucose within the normal range throughout cycles of feeding and fasting and balanced alterations in secretions of regulatory hormones( Reference Nordlie, Foster and Lange 18 , Reference Pilkis, El-Maghrabi and Claus 19 ). In contrast, older adults have a broader range over which circulating glucose is maintained and in addition have attenuated counter regulatory responses( Reference Kent 20 , Reference Kalyani and Egan 21 ). Circulating insulin levels tend to be elevated with age (approximately 8% higher than in young adults) and are indicative of reduced insulin sensitivity( Reference Melanson, Greenberg and Ludwig 22 ). Reduced insulin sensitivity or insulin resistance is a condition where individuals develop resistance to the cellular actions of insulin, characterised by an impaired ability of insulin to inhibit glucose output from the liver and to promote glucose uptake in fat and muscle( Reference Reaven 23 ). Both effects of insulin insensitivity on liver and muscle tissue cause elevations in peripheral blood glucose levels( Reference Kahn, Hull and Utzschneider 24 ). Changes in insulin action have been observed at different stages of development. Basal insulin secretion increases during puberty, falling back to pre-pubertal levels in adulthood( Reference Caprio, Plewe and Diamond 25 ). Yet, fasting glucose levels remain constant, implying an increase in tissue resistance to insulin coinciding with puberty( Reference Savage, Smith and Dunger 26 ). The reason for the puberty-induced reduction of insulin sensitivity appears to be growth-hormone related( Reference Jørgensen, Møller and Wolthers 27 – Reference Kyho and O’Sullivan 29 ). Growth hormone secretion reaches a peak at around puberty and will begin to decrease by age 21 years( Reference Savine and Sönksen 30 ). It is commonly in middle age where insulin resistance and poor glucose tolerance become a health issue( Reference Facchini, Hua and Abbasi 31 ). Given that the brain uses glucose as a primary substrate for brain function, it is perhaps not surprising that conditions that affect peripheral and central glucose regulation and utilisation may also affect cognitive functioning. Moreover, based on the evidence earlier there might be critical periods in which alterations in cerebral glucose supply might have more pronounced effects on cognitive performance.
Acute administration of a glucose load: prototypical experimental paradigm
Over the past 30 years, a large body of literature has demonstrated beneficial effects of acute glucose administration on cognition in various populations( Reference Messier 32 , Reference Smith, Riby and van Eekelen 33 ). The general methodology used in these studies involves administration of an oral glucose load (usual range between 25 and 50 g glucose) after a period of fasting (ranging from 2 h to overnight fast) followed by assessment of cognitive performance and measurement of capillary blood glucose levels( Reference Messier 32 , Reference Manning, Hall and Gold 34 ).
Using this experimental paradigm, beneficial effects have been observed across different populations. For example, glucose administration has been shown to enhance cognitive performance in adolescents( Reference Smith and Foster 35 ), young adults( Reference Sünram-Lea, Foster and Durlach 36 – Reference Foster, Lidder and Sünram 45 ), older adults( Reference Meikle, Riby and Stollery 46 , Reference Riby, Meikle and Glover 47 ) and improvements have been observed in subjects with mild or severe cognitive pathologies, including individuals with Alzheimer's disease and Down's syndrome( Reference Messier 32 , Reference Smith, Riby and van Eekelen 33 ). In addition, facilitation of cognitive performance induced by elevations in plasma glucose levels has also been reported in patients with schizophrenia( Reference Newcomer, Craft and Fucetola 48 , Reference Fucetola, Newcomer and Craft 49 ). It is important at this point to note that these results do not reflect a negative effect of fasting on cognition and memory, as the degree of fasting in which participants engaged was not exceptional and participants do not reach blood glucose levels associated with hypoglycaemia.
In terms of cognitive tasks affected, benefits have been found to occur in a range of cognitive domains, including information processing and attention( Reference Meikle, Riby and Stollery 46 , Reference Owens and Benton 50 – Reference Stollery and Christian 53 ), working memory( Reference Sünram-Lea, Foster and Durlach 36 , Reference Sünram-Lea, Foster and Durlach 37 , Reference Owen, Scholey and Finnegan 42 , Reference Kennedy and Scholey 43 , Reference Hall, Gonder-Frederick and Chewning 54 ), executive function( Reference Brandt, Gibson and Rackie 55 , Reference Benton, Owens and Parker 56 ) , problem solving( Reference Miller, Bourrasseau and Blampain 57 ) and long-term memory( Reference Sünram-Lea, Foster and Durlach 36 – Reference Sünram-Lea, Owen and Finnegan 38 , Reference Owen, Finnegan and Hu 40 – Reference Owen, Scholey and Finnegan 42 , Reference Sünram-Lea, Dewhurst and Foster 58 – Reference Sünram-Lea, Foster and Durlach 60 ). The clearest enhancement effects of increased glucose supply have been observed for long-term memory over a variety of conditions and paradigms( Reference Hoyland, Lawton and Dye 61 ). As different aspects of cognition pertain to different neural structures and networks, this allows speculation about the areas of the brain that might be particularly susceptible to glycaemic fluctuations. The robust effects on long-term memory, suggest that glucose facilitation may be particularly pronounced in tasks that pertain to the hippocampal formation( Reference Sünram-Lea, Foster and Durlach 36 ). The level of task demand is a further moderating factor for cognitive enhancement by increased glucose availability. Indeed, in younger participants, glucose-related improvement of cognition appears to be related to the difficulty of the cognitive tasks. Tasks which are more cognitively demanding appear to be more sensitive to the effect of glucose loading( Reference Sünram-Lea, Foster and Durlach 37 , Reference Kennedy and Scholey 43 , Reference Scholey, Harper and Kennedy 62 ). In addition, depletion of memory capacity and/or glucose resources in the brain due to performing a concomitant cognitive task might be crucial to the demonstration of a glucose facilitation effect( Reference Sünram-Lea, Foster and Durlach 37 ).
While both young and older adults show cognitive improvement after the oral administration of glucose, the effects appear to be more profound in older individuals( Reference Hall, Gonder-Frederick and Chewning 54 ). Cognitive decline over the aging process has been well documented( Reference Gold, McGaugh and Hankins 63 – Reference Gold and Stone 65 ). Traditionally, cognitive impairments are assumed to reflect deficits caused by damage of brain areas or systems in which cognitive processing in normal subjects occurs. However, more recently there has been a focus shift on specific physiologic and metabolic impairments that appear to contribute to the cognitive decline observed in ageing. Older adults have a broader range over which circulating glucose is maintained and in addition have attenuated counter regulatory responses( Reference Andres and Tobin 66 , Reference Frank, Roland and Sturis 67 ). These suboptimal metabolic and cognitive conditions are likely to make older individuals more susceptible to glucose facilitation of cognitive performance( Reference Messier 68 , Reference Greenwood and Winocur 69 ).
The energy cost for effortful, controlled or executive processes appears to be significantly higher than that for automatic or reflexive processes( Reference Gailliot and Baumeister 70 ). Effortful, controlled or executive processes are processes that are reliant on the central executive, in which thoughts, behaviours and actions are coordinated to allow goal directed and purposeful behaviour, while automatic and reflexive behaviours are evolutionarily predisposed or learned behaviours elicited by environmental stimuli( Reference Miller and Wallis 71 ). Indeed, lowered peripheral glucose levels following performance of a cognitively demanding task have been reported( Reference Scholey, Harper and Kennedy 62 , Reference Fairclough and Houston 72 ). This fall in plasma glucose could reflect a more efficient transfer of glucose to the brain which in turn results in increased provision centrally( Reference Scholey, Harper and Kennedy 62 ). One should be cautious when making assumptions about peripheral blood glucose levels and their putative effects on the brain, as other studies have failed to demonstrate such findings( Reference Molden, Hui and Scholer 73 , Reference Boyle, Lawton and Allen 74 ). Nevertheless, the evidence suggests that cognitively demanding tasks and in particular those relying on executive functions are sensitive to changes in glucose availability( Reference Gailliot and Baumeister 70 , Reference Kelly, Sünram-Lea and Crawford 75 ). Administration of a glucose drink would consequently provide the brain with sufficient metabolic resources for extensive cognitive processing and support the brain areas under greatest cognitive load, and thus lead to improved performance.
A further moderating factor of the impact of glucose on cognitive function is dose. As with many substances affecting cognitive performance, glucose displays an inverted U-shaped dose–response curve and its effect is time dependent( Reference Gold 76 ). For older adults 25 g glucose appear to be the optimal dose, with performance deterioration observed after administration of 75 g glucose( Reference Parsons and Gold 77 ). For young adults 25 g also seems to most reliably facilitate cognitive performance; however, there is evidence suggesting that the optimal dose or shape of the dose–response curve may be dependent on inter-individual difference in glucose metabolism, and the cognitive domain being assessed( Reference Owen, Finnegan and Hu 40 ). Of note, the cognitive enhancing effects of pharmaceutical substances such as stimulants (methylphenidate, modafinil) and acetylcholinesterase inhibitor (dementia drugs) in healthy individuals are generally moderate or small (as estimated by Cohen's d effect size) according to systematic reviews( Reference Repantis, Schlattmann and Laisney 78 , Reference Repantis, Laisney and Heuser 79 ). The effects of glucose administration are comparable with those from pharmaceutical interventions, with effect sizes for glucose effects range from 0·34 to 4·26, with typical values of 1·02, 0·81 and 1·07 for heavily loaded working memory and verbal episodic recognition and recall, respectively( Reference Riby 80 ).
Glucose facilitation of cognitive performance: putative underlying mechanisms
The precise mechanisms by which increased peripheral and/or central glucose availability affects cognitive processes are still unclear. There are two broad theoretical approaches: energetic demand models and domain specific models. Energetic demand models have their basis in the observation that the amount of mental effort involved in cognitive processing is an important determinant of a task's susceptibility to glucose enhancement( Reference Scholey, Harper and Kennedy 62 , Reference Gailliot and Baumeister 70 , Reference Fairclough and Houston 72 ). Domain specific theories, alternatively, stipulate that certain areas of the brain are more susceptible to changes in glucose availability( Reference Foster, Lidder and Sünram 45 , Reference Riby 80 , Reference Messier, Durkin and Mrabet 81 ). However, these different approaches are by no means mutually exclusive, their relative explanatory value depending on cognitive task and brain structure.
Glucose metabolism varies throughout tissue/cell types of the brain, with a clearly established correlation between increased energy metabolism and increased neuronal activity and energy metabolism( Reference Sokoloff 82 ). Both the rate of blood to brain glucose transport( Reference Lund-Andersen 83 ) and glucose metabolism( Reference Reivich, Gur and Alavi 84 ) are stimulated in different areas in the brain during cognitive tasks relevant to that area. There is evidence that performing cognitively demanding tasks increases total brain consumption by as much as 12%( Reference Madsen, Hasselbalch and Hagemann 85 ).
As described, glucose exerts quite robust effects on long-term memory tasks. The hippocampus is the brain region most strongly implicated in long-term memory performance( Reference Aggleton and Brown 86 ). Microdialysis measurements of brain glucose have shown a large decrease in hippocampal extra cellular fluid (32%) in rats tested for spontaneous alternation on a four-arm maze (a difficult memory task), while a smaller decrease (11%) was seen in rats tested on a simpler three arm-maze, suggesting that the changes observed in extra cellular fluid glucose are related to task difficulty. The fall in extra cellular fluid can be prevented by administration of glucose, which in turn leads to enhanced memory performance( Reference McNay, Fries and Gold 87 ). There is some evidence that the concentration of extracellular glucose in the brain after its transfer across the blood–brain barrier from plasma glucose varies with brain region from 1·3 mm/l in the hippocampus to 0·3–0·5 mm/l in the striatum( Reference McNay, McCarty and Gold 88 ). These findings suggest that the hippocampal area is particularly sensitive to energy fluctuations. However, the hippocampus has relatively greater glycogen stores compared with other areas suggesting that it has evolved some protection against temporary deficits (13 mm/l compared with 5–6 mm/l in the cerebral cortex( Reference Dalsgaard, Madsen and Secher 89 ).
There is evidence suggesting that the cognitive facilitation observed after glucose loading is due to an increase in enhancement of acetylcholine synthesis and/or release( Reference Messier 32 ). In addition, elevated insulin in response to hyperglycaemia rather than glucose levels per se may moderate memory performance( Reference Watson and Craft 90 ). Originally, insulin was considered only as a peripheral hormone, unable to cross the blood–brain barrier and to affect the central nervous system. However, there is now increasing evidence that neuronal glucose metabolism is antagonistically controlled by insulin and cortisol( Reference Gray, Meijer and Barrett 91 , Reference Duarte, Moreira and Oliveira 92 ).
The hippocampus, the brain region key to memory and learning, has particularly high levels of insulin receptors( Reference Marks, Porte and Stahl 93 , Reference Dore, Kar and Rowe 94 ) which are known to promote cellular glucose uptake( Reference Messier 32 , Reference Craft and Watson 95 ). Insulin-sensitive GLUT such as GLUT4 are also enriched in the hippocampus( Reference McEwen and Reagan 96 ). Given the established role of the hippocampus in memory, elevated insulin in response to hyperglycaemia may boost glucose utilisation in the hippocampus and result in improved performance( Reference Craft, Dagogo-Jack and Wiethop 97 ).
Glucose might also act via peripheral physiological mechanisms, which in turn facilitate central mechanisms involved in cognition. Messier and White( Reference Messier and White 98 , Reference White, Frederickson, McGaugh and Fenton 99 ) suggested that changes in cell membrane transport in the liver following administration of high doses of glucose and fructose (>1000 mg/kg) are detected by the coeliac ganglion, then transformed into neural signals and finally carried via the vagus nerve to the brain. In accordance with this suggestion, coeliac ganglion lesions (which block most of the efferents of the liver) have been shown to abolish the mnemonic effect of glucose( Reference White, Frederickson, McGaugh and Fenton 99 ). To date there is no concrete information available concerning how this proposed neural signal from the liver might influence cognitive performance when it reaches the brain. However, the nucleus of the solitary tract in the brain stem is the main relay station for afferent vagal nerve fibres and has widespread projections to numerous areas in the cerebral cortex, including the hippocampus and the prefrontal cortex( Reference Clark, Naritoku and Smith 100 ).
Research also shows that difficult tasks are more likely to be susceptible to glycaemic interventions. Difficult tasks include those involving executive functions pertaining to frontal brain regions: inhibition/self-control, working memory and mental flexibility( Reference Manard, Carabin and Jaspar 101 , Reference Diamond 102 ). Evidence suggested that tasks that demand such cognitive control and attentional resources appear to be more energy demanding( Reference Gailliot and Baumeister 70 ). Consequently, another area of the brain which appears to be particularly sensitive to energy fluctuations is the frontal cortex. The cerebral cortex, and in particular the prefrontal cortex, represents the neural basis of higher cognitive functions( Reference Frith and Dolan 103 , Reference Fuster 104 ). Aspects of higher-level cognition were probably one of the last cognitive abilities to develop ontogenetically. Based on the ‘last-in, first-out rule’, cognitive abilities that developed last ontogenetically are likely the first to become impaired when cognitive and/or physiological resources are compromised. Consequently, optimal performance on tasks pertaining to function of the pre-frontal cortex might require more energetic fuel than others. The research is not yet conclusive, but suggests that the underlying mechanism is multifarious. The most likely scenario is that glucose provides additional metabolic fuel under high demand conditions and that certain areas of the brain are more susceptible to limitations in fuel supply.
Glycaemic regulation and cognition
Over longer time periods, elevated blood glucose levels act as an allostatic load to biological systems and can accelerate disease processes. Chronic hyperglycaemic conditions negatively affect glycaemic regulation, i.e. the ability of the body to effectively regulate blood glucose levels and to remove glucose from the blood( Reference Eizirik, Korbutt and Hellerström 105 , Reference Marshak, Leibowitz and Bertuzzi 106 ). In addition to high carbohydrate loading, fat ingestion is also associated with development of insulin resistance through inflammation mediated mechanisms( Reference Xu, Barnes and Yang 107 ). Evidence suggests that the risk of impaired glucose regulation and type 2 diabetes is associated with a high trans fatty acid intake and a low poly-unsaturated to saturated fat intake ratio( Reference Manco, Calvani and Mingrone 108 ).
Consequently, glycaemic control is another important factor when considering cognition across the life-span( Reference Lamport, Lawton and Mansfield 109 ). Conditions in which glycaemic regulation is severely compromised are diabetes type 1 and type 2, impaired glucose tolerance and impaired fasting glucose. Cognitive impairments were indeed one of the earliest recognised neurological complications associated with diabetes( Reference Miles and Root 110 ). To date, numerous studies have compared cognitive functioning in diabetic patients with non-diabetic controls( Reference Brands, Biessels and De Haan 111 ). Although these studies differed widely with respect to patient characteristics (age, duration and type of diabetes) and cognitive tests used, the majority of these studies demonstrated cognitive impairments in this population, which included decreased performance on various attention and memory tasks( Reference Lamport, Lawton and Mansfield 109 , Reference Tun, Nathan and Perlmuter 112 – Reference Brands, Kessels and de Haan 115 ). Risk factors associated with cognitive complications in diabetes appear to be (i) degree of metabolic control( Reference Meuter, Thomas and Grüneklee 116 ) and (ii) repeated episodes of hypoglycaemia( Reference Auer 117 ). It is therefore not surprising that in children diagnosed with type 1 diabetes before age 10 years, cognitive complications are generally only observed if they have a history of hypoglycaemic seizures( Reference Kaufman, Epport and Engilman 118 ). It is evident from the literature that type 2 diabetes is the preventable metabolic condition associated with an increased risk of cognitive dysfunction( Reference Strachan, Deary and Ewing 119 – Reference Gallacher, Pickering and Elwood 121 ).
However, there is now increasing evidence of a relationship between glycaemic control and cognitive functions in non-diabetic populations( Reference Lamport, Lawton and Mansfield 109 , Reference Awad, Gagnon and Messier 113 ). Cognitive decline over the ageing process has been well documented and it has been suggested that normal ageing may represent a condition in which there is greater vulnerability to disrupted glucose regulation( Reference Gold and Stone 65 ). Indeed, evidence to support this hypothesis is provided by the finding that memory performance in elderly participants with poor glucose regulation is impaired relative to elderly participants with good glucose regulation( Reference Messier, Tsiakas and Gagnon 122 – Reference Craft, Zallen and Baker 124 ). Moreover, age-related changes in glucose metabolism have been identified as a risk factor for Alzheimer's disease( Reference Messier 32 , Reference Watson and Craft 90 , Reference Hoyer 125 ). Consistent with this notion is the finding that hyperglycaemia (induced through oral and intravenous glucose administration) can facilitate memory performance in Alzheimer's patients, at least in the early stages of the disease( Reference Craft, Asthana and Newcomer 126 ). Interestingly, alterations in blood glucose regulation seem to depend on the severity of the disease process. More specifically, high insulin levels are observable at the very early (very mild) stages and decline as dementia progresses. Moreover, memory facilitation can be achieved through glucose administration in the early stages and the degree of facilitation decreases at more advanced stages of the disease( Reference Craft, Dagogo-Jack and Wiethop 97 ). Indeed, as abnormalities in brain insulin resistance and deficiency have been observed in Alzheimer's disease, and the fact that molecular and biochemical hallmarks of Alzheimer's disease, such as neuronal loss, synaptic disconnection, tau hyperphosphorylation and amyloid-beta accumulation overlap with type 1 and type 2 diabetes, the term ‘type 3 diabetes’ has been suggested to account for the underlying abnormalities associated with Alzheimer's disease-type neurodegeneration( Reference Suzanne 127 ). A combination of diet and exercise has been demonstrated to have cognitive and metabolic benefits (improved glucose and insulin metabolism) in adults with impaired glucose tolerance( Reference Watson, Reger and Baker 128 , Reference Goodyear, Laurie and Kahn 129 ). Dietary lifestyle changes can have a positive impact throughout the lifespan and appear to not only reduce the risk of acquiring cognitive impairments, but can also attenuate existing impairments. For example, a recent study showed that a 4-week low-saturated fat/low-glycaemic index (GI) diet resulted in improved memory performance and insulin metabolism in adults with amnestic mild cognitive impairment( Reference Bayer-Carter, Green and Montine 130 ).
Perhaps more worryingly, performance decrements due to poor glucose regulation have been reported in younger individuals( Reference Lamport, Lawton and Mansfield 109 , Reference Awad, Gagnon and Messier 113 ). For example, recent studies have shown that even in a healthy young student population those with better glucose regulation (those who had the smallest blood glucose rise following glucose ingestion) perform better on tests of memory( Reference Owen, Scholey and Finnegan 42 , Reference Benton, Owens and Parker 56 , Reference Awad, Gagnon and Messier 113 , Reference Donohoe and Benton 131 – Reference Awad, Gagnon and Desrochers 133 ), vigilance( Reference Benton, Owens and Parker 56 , Reference Donohoe and Benton 131 ), planning( Reference Donohoe and Benton 131 ) and dichotic listening( Reference Parker and Benton 134 ) compared with those with poorer glucose regulation. In addition, glucose administration preferentially improved performance in those with poorer glucose regulation and the effects are less likely to be observed in good glucose regulators in both old and young populations( Reference Messier 32 ). This would suggest that glucose control or tolerance is associated with cognition throughout the lifespan. Overall there appears to be some evidence that glucoregulation may exert direct effects on cognitive function in that those with poor glucoregulation may demonstrate mild cognitive deficit compared with good glucoregulation. However, research in young adults is limited, furthermore the methodologies for determining glucoregulatory control have been varied. Only a few studies have used a standardised oral glucose tolerance test for the evaluation of glucose tolerance in healthy young adults( Reference Owen, Scholey and Finnegan 42 , Reference Donohoe and Benton 135 ). The oral glucose tolerance test involves administration of a 75 g glucose load after a minimum 8 hour fast and is the gold standard test for the diagnosis of diabetes mellitus( 136 ). Moreover, the majority of studies have only assessed one specific measurement of glucose tolerance. Several glucoregulatory indices have been previously evaluated for their relationship with cognitive performance in younger and older participants. These include: fasting levels, peak glucose levels, recovery and evoked glucose to baseline levels and incremental area under the curve( Reference Owen, Scholey and Finnegan 42 ). At a younger age, the deficits associated with poor glucoregulation may be minimal and hard to detect therefore it is important to identify the most sensitive marker. A study in our laboratory found area under the curve, which takes baseline blood glucose levels into account (area under the curve with respect to ground( Reference Pruessner, Kirschbaum and Meinlschmid 137 )), to be the best predictor of cognitive performance, whereas the most commonly used incremental area under the curve did not show a strong association( Reference Owen, Scholey and Finnegan 42 ). This suggests that overall circulating glucose levels may be an important factor in the assessment of glucoregulation in sub-clinical; populations with normal glucose tolerance as defined by the WHO( 136 ). Indeed, a recent study identified fasting blood glucose levels as a predictor for cognitive performance( Reference Hawkins, Gunstad and Calvo 138 ). Young adults who were obese but otherwise healthy had higher fasting glucose levels compared with normal weight participants. In addition, higher glucose levels were associated with poorer cognitive performance on tests of inhibitory control, especially among individuals with pre-diabetic levels. Consequently, subclinical elevations in blood glucose may contribute to cognitive impairments before the development of clinically defined disease states.
The postprandial glycaemic response and cognition
When considering the nature of glucose availability, the rate at which food increases and maintains blood glucose, i.e. ‘the GI’ appears to be an important modulating factor. Shortly after intake of a high GI food there is a relatively rapid rise in blood glucose levels followed by a corresponding rapid decrease, whereas after the intake of a low GI food there is a relatively smaller rise in blood glucose followed by more stable blood glucose concentration. GI solely provides a measure of carbohydrate quality( Reference Wolever, Vorster and Björck 139 ), whereas glycaemic load (GL) takes into account the amount of carbohydrates consumed and is calculated by multiplying the amount of available carbohydrate in a food item by the GI of the food and dividing this by 100( Reference Gilsenan, de Bruin and Dye 140 ).
Although the effect of glucose administration has been extensively studied in an acute, short-term context, much remains to be done in order to establish the cognitive effects associated with foods of low or high GI and GL. Most studies examining the effects of GI on cognition have focused on the effect of breakfast on children's cognitive performance. Children may be particularly sensitive to breakfast interventions due to their greater energetic needs during this period compared with adults( Reference Chugani 16 ). Moreover, it has been suggested that in younger children, the overnight fast induces greater metabolic stress, as the higher the ratio of brain to liver weight and the greater metabolic rate per unit of brain weight, the greater the demand on glycogen stores( Reference Pollitt, Leibel and Greenfield 141 ). It has been shown that children at risk for malnourishment have improved cognition and learning at school if provided with breakfast( Reference Hoyland, Dye and Lawton 142 ). Moreover, in developed countries it has been found that skipping breakfast can result in impaired cognitive performance( Reference Hoyland, Dye and Lawton 142 , Reference Benton and Parker 143 ). This suggests that increased plasma glucose availability due to breakfast consumption leads to better cognitive performance. Mahoney et al. ( Reference Mahoney, Taylor and Kanarek 144 ), investigated the optimal rate of glucose supply following breakfast consumption, comparing a low GI breakfast with a high GI breakfast and found that when children consumed the low GI food they remembered significantly more than when they ate the high GI breakfast. Ingwersen et al. ( Reference Ingwersen, Defeyter and Kennedy 145 ) compared the cognitive effects of a low GI breakfast and a high GI breakfast across the morning and found that performance on attention tasks was poorer 130 min after the high GI breakfast compared with the low GI breakfast. Furthermore, the low GI breakfast prevented a decline in memory performance. Overall, the results of studies assessing GI in children suggest that a lower postprandial glycaemic response may be protective against a decline in memory and attention throughout the morning( Reference Mahoney, Taylor and Kanarek 144 – Reference Young and Benton 151 ). However, the evidence is far from conclusive( Reference Brindal, Baird and Slater 152 , Reference Iovino, Stuff and Liu 153 ) and few studies have actually profiled the glycaemic response in children( Reference Brindal, Baird and Danthiir 154 ).
From a metabolic perspective, adolescence might also be a time where greater susceptibility to glycaemic variations is observed due to the specific metabolic conditions observed during that time of development( Reference Caprio, Plewe and Diamond 25 , Reference Savage, Smith and Dunger 26 ). However, few studies have looked at the effects of GI in adolescent populations and the results are somewhat contradictory. Wesnes et al. ( Reference Wesnes, Pincock and Richardson 146 ) found that a low GI breakfast resulted in better memory performance and attention, but the age range used in this study was quite large (6–16 years). Other studies found performance benefits following a high GI intervention when assessing memory performance( Reference Micha, Rogers and Nelson 149 , Reference Smith and Foster 155 ) whereas a low GI intervention proved to be beneficial for measures of attention/information processing( Reference Micha, Rogers and Nelson 149 ). Cooper et al. ( Reference Cooper, Bandelow and Nute 150 ) found no difference between high GI and low GI on reaction times, but better performance on an executive function task following low GI.
In adult populations, the outcome of investigating the effects of GI has also been somewhat inconsistent. Some show beneficial effects on cognitive performance of low-GI foods( Reference Benton, Ruffin and Lassel 147 , Reference Nilsson, Radeborg and Björck 156 , Reference Nilsson, Radeborg and Björck 157 ) whereas others show no such effects( Reference Kaplan, Greenwood and Winocur 158 , Reference Dye, Gilsenan and Quadt 159 ). Benton et al. ( Reference Benton and Jarvis 148 ) compared three breakfasts varying in GL from 2·5 to 17·86 and found that the higher GL foods led to poorer memory performance. Lamport et al. ( Reference Lamport, Dye and Mansfield 160 ) investigated the effects of low GI and high GI evening meals followed by a high GI standard breakfast on subsequent cognitive performance. Although no significant differences between evening meals on cognitive performance were observed, the high GI evening meal was associated with better memory performance the following morning after breakfast had been consumed.
To date only a few studies have been carried out into the effect of low GI and GL foods on glycaemic control and cognition in older adults, or populations with pre-existing metabolic and/or cognitive impairments. Kaplan et al. ( Reference Kaplan, Greenwood and Winocur 158 ) found no differences between meals of different GI in performance in elderly adults. Nilsson, Radeborg and Björk( Reference Nilsson, Radeborg and Björck 156 ) showed that in a sample ranging from 49 to 70 years, performance was better in the late postprandial period after consumption of a low-GI compared with a high-GI breakfast. In adults with type 2 diabetes consuming a low-GI carbohydrate meal, relative to a high-GI carbohydrate meal, has been shown to result in better cognitive performance in the postprandial period( Reference Papanikolaou, Palmer and Binns 161 ). However, two other studies by Lamport et al. ( Reference Lamport, Dye and Mansfield 160 , Reference Lamport, Lawton and Mansfield 162 ) did not find any benefits following consumption of a low GL breakfast. All of these studies investigated the acute effects of postprandial glycaemic manipulation and it may be the case that for these populations cognitive effects will only be evident with chronic improvements in glycaemic control. Indeed, dietary interventions (combined with exercise interventions) have been shown to result in improved cognitive performance in adults with impaired glucose control when they were implemented for 12 months( Reference Watson, Reger and Baker 128 ).
Overall, it appears that a quick rise in blood glucose levels has some short-term benefits, most notably on memory performance; whereas over longer periods of time (i.e. throughout the morning) a more stable blood glucose profile seems to be more beneficial. In normoglycaemic samples, effects of low GI and/or low GL foods were usually observed in the late postprandial period (75–222 min) where they seem to prevent a decline in attention and memory( Reference Mahoney, Taylor and Kanarek 144 , Reference Ingwersen, Defeyter and Kennedy 145 , Reference Benton, Ruffin and Lassel 147 ). In populations with abnormalities in glucose regulation, benefits of low GI foods have been reported in particular following longer-term intervention.
Conclusion
Based on the evidence it is clear that brain glucose utilisation changes across the lifespan, and that maintaining good glucoregulatory function and insulin sensitivity is key to promoting cognitive resilience as we age. Administration of a glucose load does not represent a viable strategy for cognitive improvement over any prolonged timeframe since consistently elevated blood glucose leads to insulin resistance. A combination of diet and exercise has been demonstrated to have cognitive and metabolic benefits (improved glucose and insulin metabolism) in adults with impaired glucose tolerance( Reference Watson, Reger and Baker 128 , Reference Goodyear, Laurie and Kahn 129 ). Dietary lifestyle changes can have a positive impact throughout the lifespan and appear to not only reduce the risk of acquiring cognitive impairments, but can also attenuate existing impairments. Although the evidence to date is promising, there is an urgent need for hypothesis driven, randomised controlled trials that evaluate the role of different glycaemic manipulations on cognition. A relatively recent review into the effects of carbohydrates on cognition in older individuals identified only one study that fulfilled these criteria( Reference Ooi, Loke and Yassin 163 ). The study that was included investigated the acute effects of a glucose drink( Reference Gagnon, Greenwood and Bherer 164 ), whereas studies investigating more complex carbohydrates were not. Future research comparing the effects of different types of carbohydrates with differing glycaemic profiles, are clearly needed. What limits our ability to draw strong conclusions from the findings of previous studies is the fact that they often differ widely with respect to subject characteristics and cognitive tests used. Future research needs to carefully consider conceptual and methodological factors including potential inter-individual differences, adequate selection of tests and control of extraneous (confounding) variables( Reference Adolphus, Bellissimo and Lawton 165 ).
Moreover carbohydrates are rarely ingested in isolation and co-ingestion of other macro-nutrients and nutritional compounds alters the rate of carbohydrate degradation during digestion and consequently affect regulation of postprandial blood glucose and insulin levels. For example, a lowering of glycaemic response has been found when purified extracts of fibre are added to a test food in sufficient quantity( Reference Jenkins, Leeds and Wolever 166 – Reference Tappy, Gügolz and Würsch 169 ). Moreover, high fibre diets have been shown to decrease postprandial blood glucose levels( Reference Post, Mainous and King 170 ), improve glycaemic control in diabetic populations and decrease the risk of type 2 diabetes( Reference Lattimer and Haub 171 , Reference Papathanasopoulos and Camilleri 172 ). Similarly, dietary proteins have been found to have positive effects on insulin production in populations with normal glucose metabolisms as well as type 2 diabetics( Reference Nilsson, Stenberg and Frid 173 – Reference Frid, Nilsson and Holst 175 ).
In conclusion, the rise in obesity, diabetes and metabolic syndrome in recent years highlights the need for targeted dietary and lifestyle strategies to promote healthy lifestyle and brain function across the lifespan and for future generations. The data indicate that modifiable lifestyle factors and most notably dietary changes may contribute significantly to optimal cognition across the lifespan. Consequently, the therapeutic effects of longer-term dietary intervention may be a promising avenue of exploration. Lifestyle changes are difficult to execute and to maintain, but present an exciting potential for optimising cognitive performance across the lifespan.
Acknowledgements
The authors are grateful to the organisers of the Joint Winter Meeting 2016 between the Nutrition Society and the Royal Society of Medicine for the invitation to present this paper.
Financial Support
None.
Conflicts of Interest
S. I. S-L. and L. O. received no support from any organisation for the submitted work but have previously received research funding from the food and drinks industry.
Authorship
S. I. S-L. drafted, revised and presented the paper. O. L. participated in writing and revising the manuscript.




