Subaortic stenosis was defined as obstruction of the left ventricular outflow tract below the level of the aortic valve. Subaortic stenosis includes numerous anatomical variants. Subaortic stenosis can be caused by a simple localised or annular subvalvular membrane septum, or by a malapposition of the conoconus septum.Reference Benqing, Kai, Sen, Jun, Zhongdong and Shoujun1 At the same time, factors such as ventricular septal hypertrophy due to hypertrophic obstructive cardiomyopathy or systolic forward motion of the mitral valve can lead to subaortic stenosis. Among the many causes of left ventricular outflow tract obstruction, the incidence of subaortic stenosis can reach 8–30%.Reference Laredo, Khraiche and Raisky2 Among them, localised subaortic stenosis is about 70%, and diffuse stenosis is about 12%.Reference Pickard, Geva, Gauvreau, Del and Geva3–Reference Laguna, Blanco and Carrascal5 In addition, subaortic stenosis is often associated with complex cardiac anomalies such as interrupted aortic arch, transposition of the great arteries, aortic valve stenosis or aortic regurgitation. Turbulence and jet flow may damage the aortic leaflets and predispose them to regurgitation and infection. There may be thick fibrous tissue on the left ventricular surface of the aortic valve leaflet, and the valve may be deformed, resulting in aggravation of aortic insufficiency.Reference Parry, Kovalchin and Suda6 There are few reports on the mid- and long-term outcomes of surgical treatment of subaortic stenosis in the published literature, and most of them focus on the early outcomes. This article summarised the clinical data of surgical treatment of subaortic stenosis in our centre in the past 12 years and analysed the early and long-term efficacy of different surgical techniques for the treatment of subaortic stenosis. In this study, we systemically reviewed our experience in the optimal surgical strategy for patients with subaortic stenosis, aiming to provide evidence for clinical decision-making.
Materials and methods
Study design
This study was approved by our Institutional Ethics Review Management Committee, and because it was a retrospective study, patients' informed consent was waived. The clinical data of 90 patients who underwent surgical repair of subaortic stenosis in Beijing Fuwai Hospital from December 2010 to December 2022 were retrospectively analysed. Inclusion criteria: (1) patients with isolated subaortic stenosis; (2) CHD with subaortic stenosis; (3) patients with new or recurrent subaortic stenosis after CHD operation; (4) patients with drug-refractory hypertrophic obstructive cardiomyopathy. The patients were divided into group A and group B according to whether left ventricular outflow tract muscle was removed or not. There were 50 patients in group A, all with a discrete membrane subaortic stenosis. There were 40 patients in group B, including 34 patients with diffuse subaortic stenosis and seven patients with a discrete membrane subaortic stenosis. Perioperative clinical data and postoperative follow-up data of all children were collected from the ward medical record system, outpatient medical record system, and telephone follow-up in our hospital. Patients underwent outpatient review at 1, 3, 6, and 12 months in the first postoperative year and annually thereafter. Echocardiography chest X-ray and electrocardiogram were routinely performed during follow-up.
Definition and surgical indications
Early death was defined as death within 30 days after surgery or before discharge. Late mortality was defined as death that occurred after discharge from the hospital. Left ventricular outflow tract re-obstruction is defined as a left ventricular outflow tract pressure gradient > 40 mm Hg on follow-up ultrasound. Surgical indications: (1) subaortic stenosis patients with symptoms of chest tightness, chest pain, exertional shortness of breath, or syncope; (2) new-onset aortic regurgitation; (3) for patients with hypertrophic obstructive cardiomyopathy or asymptomatic left ventricular outflow tract stenosis, peak left ventricular outflow tract pressure gradient at rest or under stress > 50 mm Hg; (4) refractory hypertrophic obstructive cardiomyopathy; (5) left ventricular outflow tract stenosis caused by subaortic membrane stenosis, ventricular septal shift, and abnormal attachment of submitral tissue; and (6) left ventricular outflow tract pressure gradient > 70 mm Hg in patients with diffuse subaortic stenosis.
Surgery
All patients in the group were placed in the supine position, with a median sternal incision and routine establishment of medium and low-temperature cardiopulmonary bypass. (1) Simple membrane resection: After cardiac arrest, a transverse incision was established at the sinus tube junction above the ascending aorta to expose the aortic valve. If it was difficult to expose the aortic valve, the aortic incision could be extended to the midpoint of the noncoronary sinus annulus to explore the development and function of the aortic valve and fully expose the membrane septum. The location, extent of involvement, and adjacent relationship to the aortic and mitral valves of the membrane were evaluated. The membrane tissue was suspended and pulled with 6-0 Prolene suture, and the portion of the membrane attached to the muscle was mostly blunt dissection. The membrane portion extending to the septal septum, mitral valve leaflet, and aortic valve leaflet was removed sharply to avoid damage to the conduction bundle or leaflet tissue. (2) Membrane and muscle resection: On the basis of simple diaphragm resection, the hypertrophic myocardium was symmetrically resected from the middle part below the right coronary valve to the mitral valve root below the left coronary valve in the groove pattern, centreing on the junction between the left coronary valve and the right coronary valve. The depth of the groove should be individualised according to the preoperative colour Doppler ultrasound and CT. Damage to the myocardial conduction bundle and mitral valve should be avoided during the operation. (3) Modified Konno operation: The position of the aortic annulus and membrane was detected by right-angle clamp through the aortic incision. The ventricular septum was incised through the right ventricular outflow tract, and the subaortic fibrous membrane was fully exposed. The hypertrophic myocardium of the left ventricular outflow tract was removed through a septal incision. The extent of resection was up to the level of the left and right junction of the aortic annulus and down to the level of the papillary muscles. At the same time, intracardiac malformation correction was performed. Ventricular septal defects were sutured intermittently with glutaraldehyde-treated autologous pericardium, dacron patch or bovine pericardium patch to reduce the occurrence of residual shunt. The right ventricular outflow tract incision was closed by patch or direct suture according to the intraoperative situation.
Statistical analyses
The normality of the distribution was tested using the Shapiro–Wilk test. The normally distributed measurement data were expressed as mean±standard deviation, and the t-test was used for comparison between groups; the non-normally distributed measurement data were expressed as median (interquartile range), and the Wilcoxon–Mann–Whitney U-test was used for comparison between groups. Descriptive statistics for categorical data were reported as frequency/percentage and were compared using the Pearson’s χ2 or Fisher’s exact test. Freedom from time-dependent endpoints was studied using Kaplan–Meier and Log-rank test. Cumulative survivals and freedom from reoperation were visualised using Kaplan–Meier curves. All statistical analyses were performed by IBM SPSS Statistics for Windows, version 27.0 (IBMCorp., Armonk, N.Y., USA), and p ≤ 0.05 was considered statistically significant.
Results
Patient characteristics
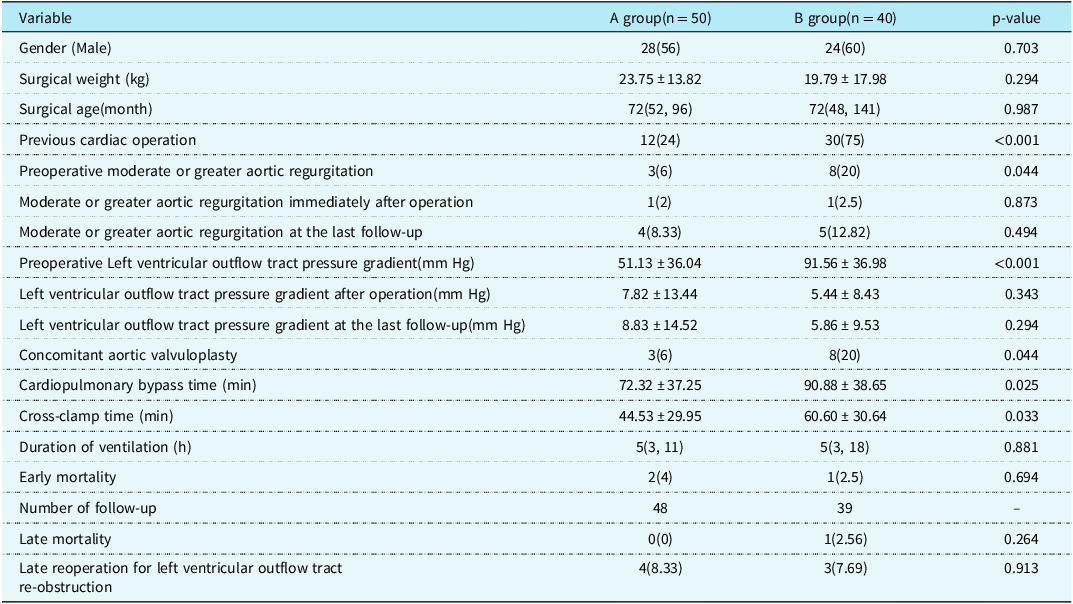
There were 55 males and 35 females with a median age of 72 (46,132) months and an average surgical weight of (21.35 ± 15.84) kg. The preoperative left ventricular outflow tract pressure gradient was (70.16 ± 41.02) mm Hg, and 55 patients had aortic regurgitation before operation. Forty-eight patients were treated for the first time for subaortic stenosis. Forty-two patients had a history of previous cardiac operation, including 11 cases of ventricular septal defect repair, 24 cases of double outlet right ventricle repair, five cases of partial atrioventricular septal defect repair, and two cases of subaortic membrane resection. Group A included 50 patients with subaortic stenosis who underwent simple membrane resection, and group B included 40 patients with subaortic stenosis who underwent fibrous membrane and muscle resection or modified Konno procedure. The preoperative left ventricular outflow tract pressure gradient in group A was (51.13 ± 36.04) mm Hg. The mean preoperative left ventricular outflow tract pressure gradient in group B was (91.56 ± 36.98) mm Hg, which was higher than that in group A (p < 0.001). See Table 1.
Table 1. Comparison of clinical data between the two groups [Mean ± SD/ case (%) /M (P25, P75)].
Early outcomes
The left ventricular outflow tract pressure gradient was (6.58 ± 12.35) mm Hg immediately after operation. The left ventricular outflow tract pressure gradient in group A was (7.82 ± 13.44) mm Hg immediately after operation. The left ventricular outflow tract pressure gradient in group B was (5.44 ± 8.43) mm Hg immediately after operation, and the difference was not statistically significant (p = 0.343). The cardiopulmonary bypass time of groups A and B were (72.32 ± 37.25) min and (90.88 ± 38.65) min, respectively, and aortic cross-clamp time were (44.53 ± 29.95) min and (60.60 ± 30.64) min, respectively. The cardiopulmonary bypass time (p = 0.025) and aortic cross-clamp time (p = 0.033) in group B were significantly longer than those in group A. There was one case of delayed sternal closure in group A and two cases in group B. One patient in group B was implanted with a permanent pacemaker due to degree III atrioventricular block. One patient in group A underwent reexploration due to pericardial effusion. There were three early deaths. Two patients in group A underwent simple membrane resection and concomitant mitral valvuloplasty, of which one patient died of cardiac arrest caused by malignant ventricular arrhythmia 3 days after operation, and the other patient died of heart failure 7 days after operation. One patient in group B died of severe pneumonia infection 28 days after operation.
Late outcomes
The median follow-up time was 60 (12,125) months. During the follow-up, one patient in group B died suddenly 1 year after operation, and the other patients were still alive during the follow-up. The postoperative survival curve was drawn with death as the end point and postoperative time as the time variable. The 1 -, 5 -, and 12-year survival rates of the whole group were 95.5%, 95.5%, and 95.5%, respectively, as shown in Figure 1a. The 1 -, 5 -, and 12-year survival rates of group A were 96.0%, 96.0%, and 96.0%, respectively, and those of group B were 94.7%, 94.7%, and 94.7%, respectively (log-rank p = 0.830), as shown in Figure 1b. Seven patients had left ventricular outflow tract re-obstruction during follow-up. There were four patients in group A and three patients in group B who underwent reoperation due to left ventricular outflow tract stenosis. The rate of freedom from reoperation for left ventricular outflow tract re-obstruction was drawn with the follow-up time as the time variable. Freedom from reoperation was 98.8%, 89.7%, and 85.0% at 1, 5, and 12 years, respectively, as shown in Figure 2a. Freedom from reoperation at 1, 5, and 12 years was 97.8%, 89.0%, and 89.0% in group A, and 100.0%, 90.7%, and 81.6% in group B, respectively. There was no significant difference in freedom from reoperation for left ventricular outflow tract re-obstruction between groups A and B (log-rank, p > 0.05. p = 0.904), as shown in Figure 2b. The left ventricular outflow tract pressure gradient in group A was (8.83 ± 14.52) mm Hg at the last follow-up. The left ventricular outflow tract pressure gradient in group B was (5.86 ± 9.53) mm Hg at the last follow-up, and the difference was not statistically significant (p = 0.294), as shown in Table 1.

Figure 1. a Overall survival rate. b Survival rate in different subgroups.
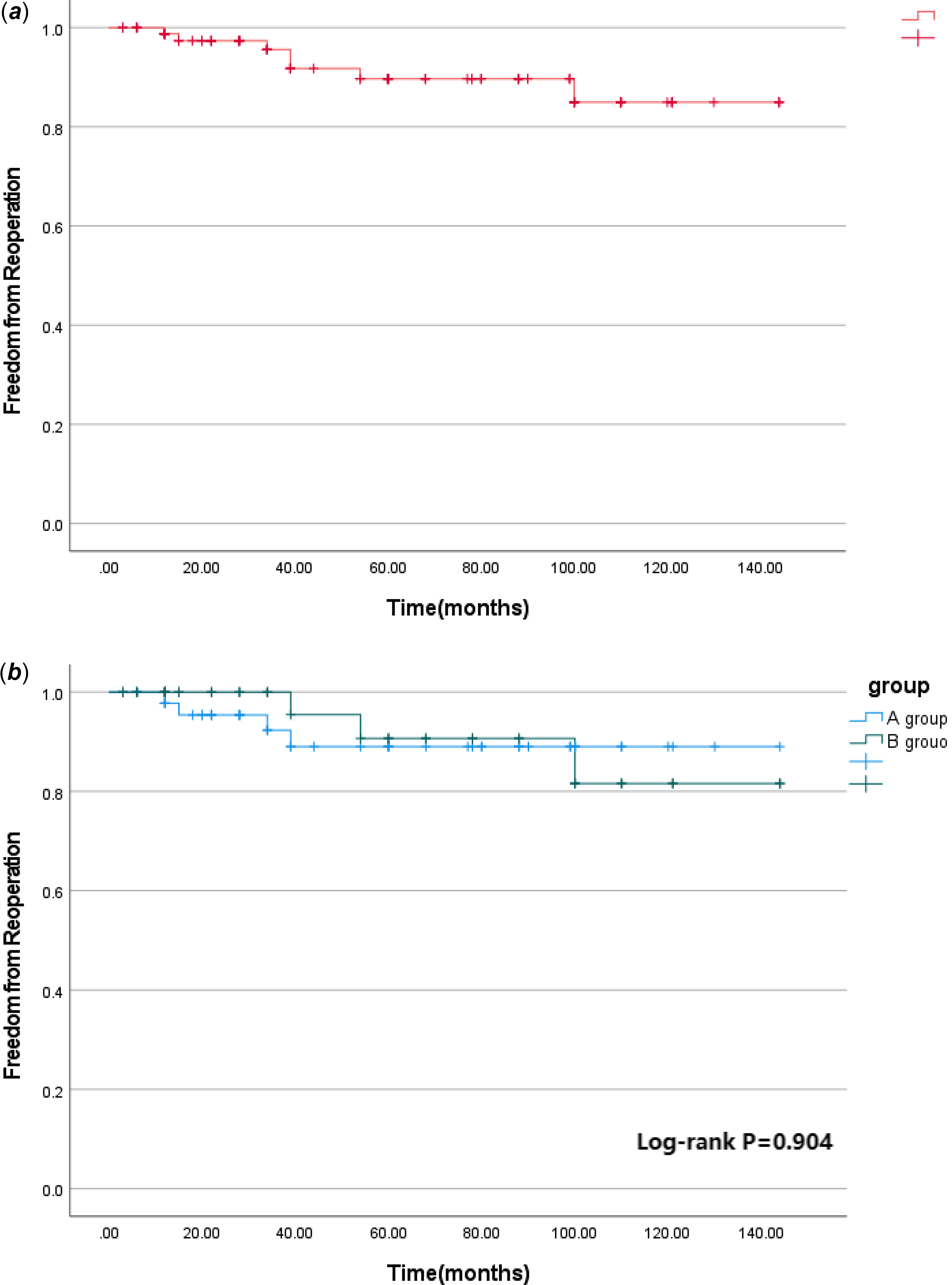
Figure 2. a Freedom from reoperation for late left ventricular outflow tract obstruction in the whole cohort. b Freedom from reoperation for late left ventricular outflow tract obstruction in different subgroups.
Moderate or greater aortic regurgitation
There were 11 cases of moderate or greater aortic regurgitation before operation, including three cases in group A and eight cases in group B. The proportion of moderate or greater aortic regurgitation in group B was higher than that in group A (p = 0.044). All 11 patients underwent aortic valvuloplasty during the operation. Intraoperative oesophageal ultrasound showed that there was no significant difference in group A and one case in group B with moderate or greater aortic regurgitation immediately after operation (p = 0.873). Long-term ultrasound follow-up showed that four cases in group A and five cases in group B had moderate or greater aortic regurgitation, and the difference was not statistically significant (p = 0.494). See Table 1 and Figure 3. In group A, there was no significant improvement in preoperative moderate or greater aortic regurgitation after operation (p = 0.307), and no significant aggravation was found in long-term follow-up compared with the results after operation (p = 0.154), see Figure 4a. In group B, the preoperative moderate or greater aortic regurgitation was significantly improved after operation (p = 0.013), and the long-term follow-up showed that there was no significant aggravation compared with the results after operation (p = 0.083), see Figure 4b.
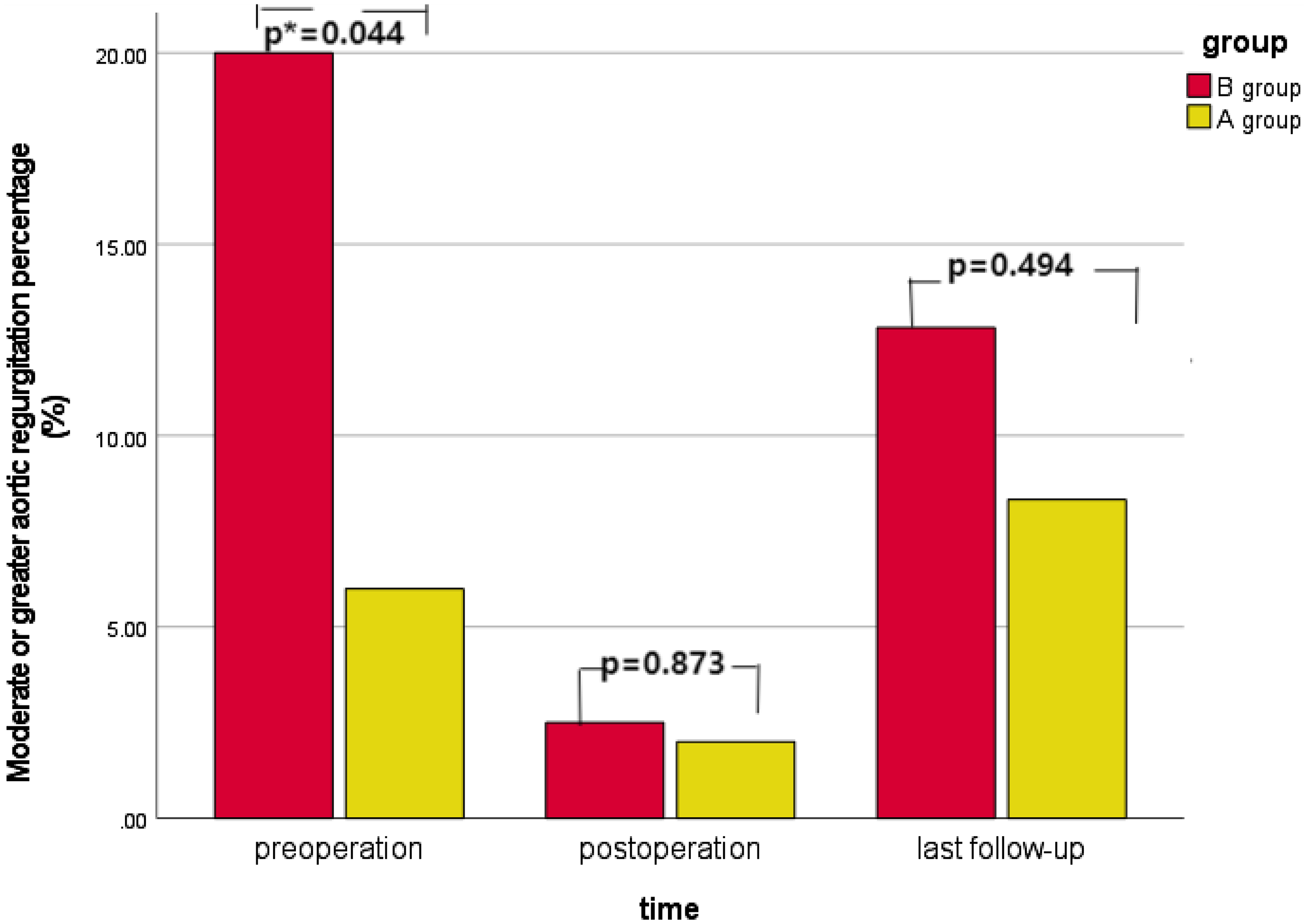
Figure 3. Changes in the proportion of moderate or greater aortic regurgitation in different subgroups over time.
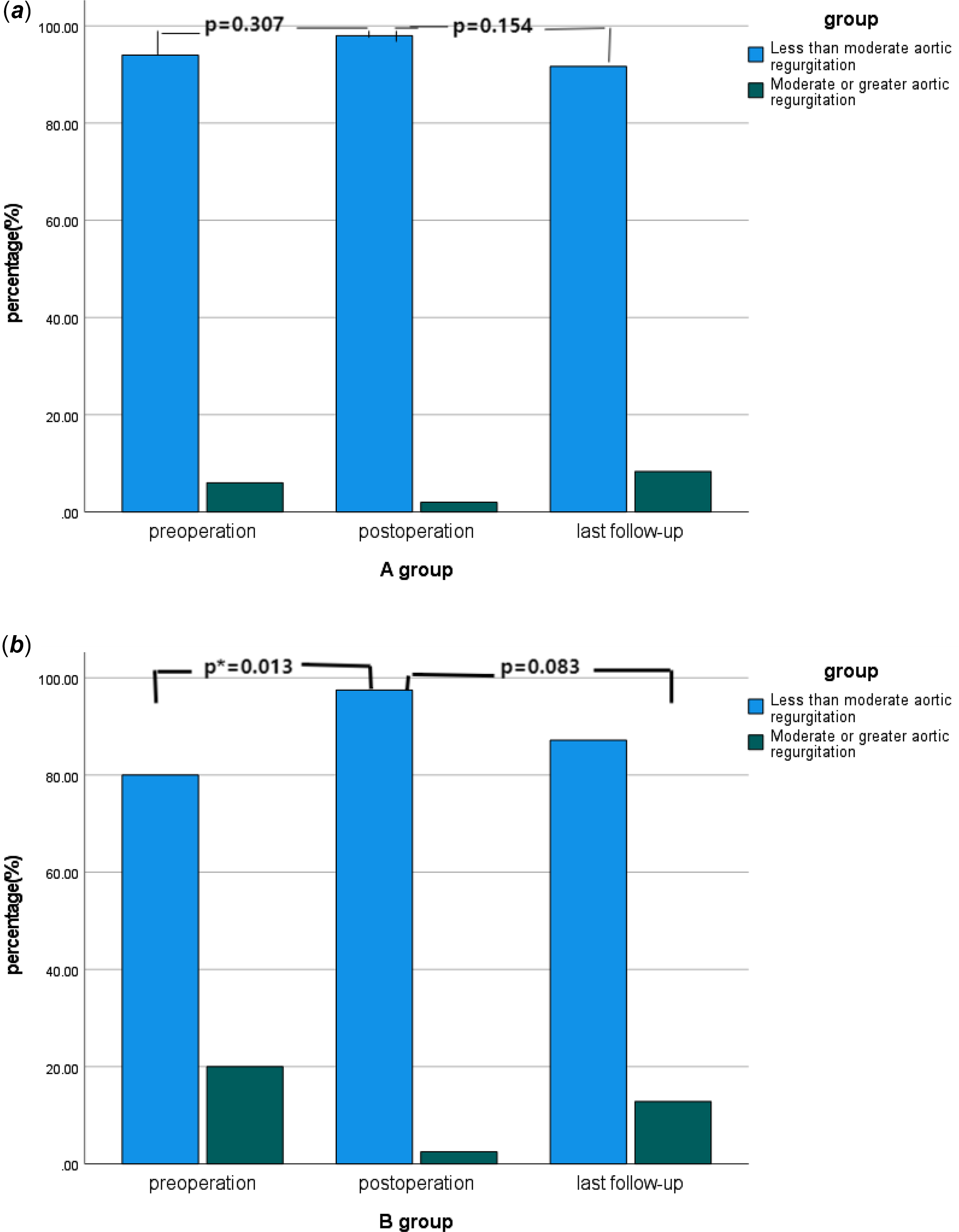
Figure 4. a Changes in the proportion of moderate or greater aortic regurgitation in A group over time. b Changes in the proportion of moderate or greater aortic regurgitation in B group over time.
Discussion
Subaortic stenosis in children is a relatively complex type of congenital left ventricular outflow tract obstruction, which is often combined with multilevel compound obstruction, mitral valve disease, aortic arch disease, and left ventricular endocardial fibroelastosis.Reference Shuo, Jun and Shoujun7 Left ventricular outflow tract obstruction is caused by a variety of reasons such as abnormal fibrous hyperplasia under the aortic valve, abnormal subvalvular tissue attachment, ventricular septal shift, and muscle hypertrophy. Subaortic stenosis is mainly characterised by increased left ventricular ejection resistance, left ventricular wall myocardial hypertrophy, subendocardial myocardial ischaemia, and fibrosis, which in turn leads to aortic valve stenosis or insufficiency and ascending aortic dilatation.Reference Geva, McMahon, Gauvreau, Mohammed, Del and Geva8 Most patients with mild subaortic stenosis have no clinical symptoms. However, patients with severe stenosis may have clinical symptoms such as decreased activity tolerance, chest tightness, shortness of breath, chest pain, syncope, and even sudden death. In addition, subaortic stenosis is often combined with other cardiac malformations such as aortic stenosis or regurgitation and mitral regurgitation.Reference Donald, Naimo and D'Udekem9–Reference Dorobantu, Sharabiani and Martin11 Subaortic stenosis can be divided into localised lesions and diffuse lesions according to the pathological characteristics of subaortic stenosis. The degree of subaortic stenosis is different, so it is very important to choose the appropriate surgical method. According to the experience of our centre, for patients with localised left ventricular outflow tract stenosis with subaortic septum and local muscle hyperplasia, simple membrane resection is usually used to relieve left ventricular outflow tract stenosis. However, for patients with diffuse subaortic stenosis caused by extensive fibrous tissue hyperplasia or myocardial thickening of the left ventricular outflow tract, membrane and muscle resection or modified Konno procedure are mostly selected in our centre to fully remove the muscle and fibrous tissue of the left ventricular outflow tract to ensure the patency of the left ventricular outflow tract. Especially in infants and young children, due to poor surgical exposure and uncertain surgical effect, the postoperative mortality and restenosis rates are higher than those in other age groups. It has been reported by other foreign centresReference Laguna, Blanco and Carrascal5,Reference Mashari and Mahmood12,Reference Dearani13 that the long-term survival rate of patients with subaortic stenosis after operation is about 93%–99.6%. The 12-year survival rate in our study was 95.5%, which was similar to that in other centres.
Left ventricular outflow tract re-obstruction is an important factor leading to reoperation in patients with subaortic stenosis.Reference Dearani13–Reference Stauber, Wey and Greutmann15 Previous studies at home and abroad have reported the rate of re-intervention after operation. Pickard et al.Reference Pickard, Geva, Gauvreau, Del and Geva3 found that 15.6% of patients required re-intervention of the left ventricular outflow tract, and the rate of re-intervention of the left ventricular outflow tract was 8.7% and 23.3% 5 and 10 years after the first resection, respectively. However, in a study involving 83 patients with subaortic stenosis, Valeske et al.Reference Valeske, Yerebakan, Mueller and Akintuerk16 found that about 46% of patients with diffuse subaortic stenosis required re-intervention for subaortic stenosis. During a median follow-up of 60 (12, 125) months, seven patients (8.0%) underwent reoperation for subaortic stenosis, and the results were better than previously reported. Therefore, for patients with diffuse subaortic disease, extended resection of the left ventricular outflow tract muscle or left ventricular outflow tract plasty is recommended to obtain a good long-term prognosis. In addition, left ventricular outflow tract re-obstruction is caused by a variety of factors. Studies at home and abroad generally believe that the age of first operation, the extent of left ventricular outflow tract stenosis, left ventricular outflow tract pressure gradient, and aortic valve stenosis are important risk factors affecting the prognosis. Preoperative left ventricular outflow tract pressure gradient ≥ 60 mm Hg is an independent risk factor for reoperation. In a retrospective study with a median follow-up of 8.2 years, Geva et al.Reference Geva, McMahon, Gauvreau, Mohammed, Del and Geva8 found that patients with a preoperative left ventricular outflow tract pressure gradient ≥ 60 mm Hg had a 4.2-fold higher risk of left ventricular outflow tract restenosis than other patients. However, Pickard et al.Reference Pickard, Geva, Gauvreau, Del and Geva3 believed that patients with preoperative left ventricular outflow tract pressure gradient ≥ 60 mm Hg had only 2.2 times the risk of left ventricular outflow tract re-intervention compared with other patients. Progressive left ventricular outflow tract restenosis is generally not accompanied by clinical symptoms. However, with the increase of the severity of left ventricular outflow tract obstruction, patients gradually have decreased exercise tolerance and even sudden syncope. The best way to diagnose progressive left ventricular outflow tract restenosis is continuous and regular postoperative Doppler echocardiography. Transthoracic echocardiography is the main method for the diagnosis of progressive left ventricular outflow tract restenosis, which can accurately identify the location of restenosis, left ventricular outflow tract obstruction, left ventricular hypertrophy, left ventricular systolic function, diastolic function, and so on. During the follow-up of this study, seven patients with left ventricular outflow tract restenosis underwent reoperation to relieve obstruction, and they are still under follow-up, and no secondary obstruction occurred.
At present, the clinical surgical strategy of subaortic stenosis is still controversial.Reference Dearani13,Reference Tanaka, Miyamoto, Minami and Miyaji17 Some scholarsReference Sarioglu, Arnaz and Saygili14,Reference Backer, Eltayeb and Monge18 believe that after simple membrane resection, left ventricular outflow tract restenosis will occur rapidly, which will increase the probability of re-intervention, and affect the function of the aortic valve and cause aortic regurgitation. However, according to our experience, different surgical strategies should be selected according to the anatomical lesions of the left ventricular outflow tract before operation, and similar mid- and long-term outcomes can be achieved. In this study, the degree of left ventricular outflow tract stenosis in group B was more severe than that in group A before operation, but the patients in group A and group B obtained ideal and similar results during the follow-up.
Aortic regurgitation is another important factor leading to reoperation, and different left ventricular outflow tract surgical strategies have a significant impact on postoperative aortic valve function.Reference Benqing, Kai, Sen, Jun, Zhongdong and Shoujun1 There were 11 patients (12.22%) with moderate or greater aortic regurgitation assessed by preoperative ultrasound, and all of them underwent aortic valvuloplasty during the operation. Aortic valvuloplasty consisted of thinning of the thickened aortic leaflets and 7–0 suture of the leaflets. In this study, the proportion of patients with moderate or greater aortic regurgitation in group B was higher than that in group A before operation, but similar results were obtained immediately after operation and during follow-up. In group B, moderate and greater aortic regurgitation improved significantly immediately after operation, and there was no significant aggravation in long-term follow-up compared with the results immediately after operation. In group A, there was no significant improvement in aortic regurgitation after operation and long-term follow-up. Therefore, for patients with moderate or severe aortic regurgitation before operation, we recommend that simultaneous aortic valvuloplasty should be performed during extended left ventricular outflow tract resection, which is beneficial to improve postoperative aortic valve function and reduce the incidence of late aortic regurgitation.
Limitations
The most important limitations of this study are its retrospective and single-institution nature. An additional limitation is the relatively small study size limiting the statistical robustness of any inference that may be drawn. In the future, longer follow-up and larger sample size multicentre clinical studies are needed to further evaluate the long-term outcomes. Despite these limitations, this is the largest single-centre cohort study with the longest follow-up in China to determine the optimal surgical strategy for subaortic stenosis, which helps to provide evidence for clinical decision-making.
Conclusions
According to the different anatomical lesions of left ventricular outflow tract, the individualised surgical treatment strategy for patients with subaortic stenosis can achieve good long-term results. The long-term survival rate and freedom from reoperation due to left ventricular outflow tract restenosis were comparable between simple membrane resection and extended left ventricular outflow tract resection. For patients with moderate or greater aortic regurgitation before extended left ventricular outflow tract resection, simultaneous aortic valvuloplasty is beneficial to improve postoperative aortic valve function.
Data availability statement
All data are incorporated into the article, and the data underlying this article are available in the article.
Acknowledgements
We acknowledge the roles of our colleagues, perfusionists, nurses, and others involved in the care of the study participants.
Author contribution
Zhangwei Wang: Conceptualisation; Data curation; Formal analysis; Investigation; Methodology; Software; Validation; Visualisation; Writing—original draft. Kai Ma: Writing—review & editing. Yaobin Zhu: Writing—review & editing. Zhiqiang Li: Writing—review & editing. Shoujun Li: Writing—review & editing.
Financial support
None.
Competing interests
None.








