The study of first-episode psychosis (FEP) patients is of particular interest because it allows the investigation of neurobiological processes, ruling out the effects of long-term medications and chronicity (Squarcina et al. Reference Squarcina, Perlini, Peruzzo, Castellani, Marinelli, Bellani, Rambaldelli, Lasalvia, Tosato, De Santi, Spagnolli, Cerini, Ruggeri and Brambilla2015). Psychotic disorders are highly debilitating, with symptoms ranging from delusions and hallucinations to cognitive deficits (Altamura et al. Reference Altamura, Buoli, Caldiroli, Caron, Cumerlato Melter, Dobrea, Cigliobianco and Zanelli Quarantini2015). In this context, Proton Magnetic Resonance Spectroscopy (¹H MRS) is the only non-invasive imaging technique that can quantify the concentration of relevant biochemicals in vivo in localised brain areas such as the anterior cingulate cortex (ACC) (Benes & Berretta, Reference Benes and Berretta2001; Stanley, Reference Stanley2002; Brambilla et al. Reference Brambilla, Stanley, Nicoletti, Sassi, Mallinger, Frank, Kupfer, Keshavan and Soares2005). The ¹H metabolites that are commonly reported include N-acetylaspartate (NAA), a marker of functioning neurons (Stanley et al. Reference Stanley, Vemulapalli, Nutche, Montrose, Sweeney, Pettegrew, MacMaster and Keshavan2007), phosphocreatine plus creatine (PCr + Cr), involved with energetic processes, glycerophosphocholine plus phosphocholine (GPC + PC), catabolic and anabolic metabolites of membrane phospholipids, and neurotransmitters, glutamine, glutamate and gamma amino-butyric acid (GABA). ¹H MRS has been largely employed in psychosis with meta-analyses showing, in general, decreased NAA levels in schizophrenia in prefrontal and temporal areas (Kraguljac et al. Reference Kraguljac, Reid, White, Jones, den Hollander, Lowman and Lahti2012), while glutamate has been found to be altered in schizophrenia and in individuals with a high risk of developing the disease, especially in thalamus and prefrontal regions (Marsman et al. Reference Marsman, van den Heuvel, Klomp, Kahn, Luijten and Hulshoff Pol2013; Merritt et al. Reference Merritt, McGuire and Egerton2013). The investigation of brain metabolites can therefore be of help in shedding light on the aetiology and biological mechanisms of psychosis.
Neuroanatomical models of schizophrenia are in agreement with the hypothesis of a frontal lobe biochemical alteration (Zabala et al. Reference Zabala, Sánchez-González, Parellada, Moreno, Reig, Burdalo, Robles, Desco and Arango2007). Thus, the study of prefrontal integrity and function in the early stages of the disease is of particular interest. The cingulate cortex, which is part of the cortico-striato-thalamo-cortical networks, is involved in various cognitive control and emotional processes. In particular, the evidence is compelling in implicating the ACC in the pathogenesis of schizophrenia (Baiano et al. Reference Baiano, David, Versace, Churchill, Balestrieri and Brambilla2007), particularly concerning negative symptoms (Hardy et al. Reference Hardy, Tal, Babb, Perry, Messinger, Antonius, Malaspina and Gonen2011; Bersani et al. Reference Bersani, Minichino, Fojanesi, Gallo, Maglio, Valeriani, Biondi and Fitzgerald2014). The neurobiology underlying psychosis is still unclear: MRS techniques could allow the identification of changes such as neuroplasticity or neuropil loss (Théberge et al. Reference Théberge, Bartha, Drost, Menon, Malla, Takhar, Neufeld, Rogers, Pavlosky, Schaefer, Densmore, Al-Semaan and Williamson2002). In this review, we consider studies, which focus on brain metabolites in the ACC of FEP patients measured with 1H MRS. A bibliographic search on PUBMED on MRS studies exploring ACC in FEP was performed. The search terms used to identify the articles of interest were ‘MRS’, ‘spectroscopy’, ‘anterior cingulate’, ‘first episode psychosis’. Ten papers, which have been summarised in Table 1, met these inclusion criteria.
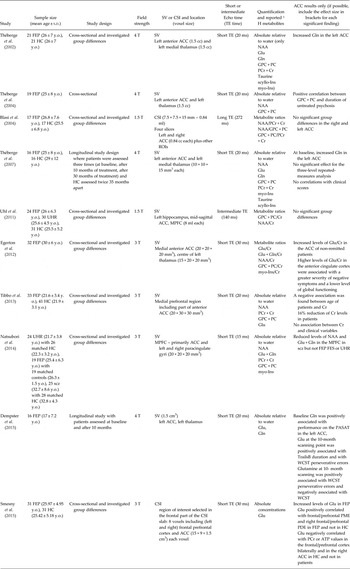
Table 1. Selection of studies on first-episode psychosis investigating anterior cingulate cortex metabolism with 1-H magnetic resonance spectroscopy

MRS, magnetic resonance spectroscopy; FEP, first episode psychosis; SV, single voxel; CSI, chemical shift imaging; HC, healthy controls; SCZ, schizophrenia; WM, white matter; GM, gray matter; CSF, cerebro-spinal fluid; ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; MPFC, medial prefrontal cortex; NAA, N-acetylaspartate; NAAG, N-acetylaspartylglutamate; Gln, glutamine; Glu, glutamate; Glc, glucose; PCr + Cr, phosphocreatine + creatine; GPC + PC, glycerophosphocholine + phosphocholine; mI, myo-Inositol; myo-Ins, myo-inositol; scyllo-Ins, scyllo-Inositol; TE, echo time; y.o., years old.
Four out of the ten studies reported increased glutamatergic function (considered as glutamate, glutamine or glutamate plus glutamine) in FEP, whereas four studies showed preserved levels. Just one study reported changes in creatine (Tibbo et al. Reference Tibbo, Bernier, Hanstock, Seres, Lakusta and Purdon2013). Going into details, Thèberge et al. (Reference Théberge, Bartha, Drost, Menon, Malla, Takhar, Neufeld, Rogers, Pavlosky, Schaefer, Densmore, Al-Semaan and Williamson2002) found higher levels of glutamine, and no other metabolites, in the left anterior ACC of FEP, in accordance with the hypothesised abnormal glutamatergic activity in schizophrenia (Marsman et al. Reference Marsman, van den Heuvel, Klomp, Kahn, Luijten and Hulshoff Pol2013). These results in FEP were confirmed in a 2007 study (Théberge et al. Reference Théberge, Williamson, Aoyama, Drost, Manchanda, Malla, Northcott, Menon, Neufeld, Rajakumar, Pavlosky, Densmore, Schaefer and Williamson2007): interestingly, glutamine levels did not decrease after 30 months of follow-up, suggesting that the lower levels found in chronic illness by the same group (Théberge et al. Reference Théberge, Al-Semaan, Williamson, Menon, Neufeld, Rajakumar, Schaefer, Densmore and Drost2003), may need more time to appear. An association between glutamine and FEP has been highlighted also by the fact that its levels were found to be associated with patient performance in neuropsychological tests, such as the Wisconsin Card Sorting Test, the Paced Auditory Serial Addition Task and the Trail Making Test B (Dempster et al. Reference Dempster, Norman, Théberge, Densmore, Schaefer and Williamson2015). An involvement of the glutamatergic function in the early stage of disease in frontal regions has also been confirmed by several studies, which found altered levels of glutamate. In particular, increased glutamate levels were reported in prefrontal areas of FEP (Smesny et al. Reference Smesny, Gussew, Biesel, Schack, Walther, Rzanny, Milleit, Gaser, Sobanski, Schultz, Amminger, Hipler, Sauer and Reichenbach2015), including the anterior ACC. Smesny et al. (Reference Smesny, Gussew, Biesel, Schack, Walther, Rzanny, Milleit, Gaser, Sobanski, Schultz, Amminger, Hipler, Sauer and Reichenbach2015) hypothesised that glutamate is linked with cellular energy and membrane lipids metabolism, suggesting a relationship with membrane atypical behavior. This indicates that medication targeting neuroprotection could be of great importance especially at the early stages of disease. Levels of glutamate were found to be higher in non-remitted patients in respect to remitted patients, when compared after antipsychotic treatment, and to be associated with lower functioning and worse negative symptoms (Egerton et al. Reference Egerton, Brugger, Raffin, Barker, Lythgoe, McGuire and Stone2012). This indicates that the clinical status of patients could be related with glutamatergic dysfunction, which might then be targeted in patients, especially those not responding to antipsychotics. Results on glutamatergic function in FEP are not always in agreement, as some studies did not find differences between groups (Galinska et al. Reference Galinska, Szulc, Tarasow, Kubas, Dzienis, Czernikiewicz and Walecki2009; Tibbo et al. Reference Tibbo, Bernier, Hanstock, Seres, Lakusta and Purdon2013). This could be related to the exact positioning of the voxel in the ACC. For example, Tibbo et al. (Reference Tibbo, Bernier, Hanstock, Seres, Lakusta and Purdon2013) positioned the voxel in the medial prefrontal region, including only part of anterior ACC. All studies considering NAA levels did not find any difference in the ACC of FEP compared with healthy subjects, even when finding differences in chronic schizophrenia patients (Natsubori et al. Reference Natsubori, Inoue, Abe, Takano, Iwashiro, Aoki, Koike, Yahata, Katsura, Gonoi, Sasaki, Takao, Kasai and Yamasue2014), possibly indicating that neuronal integrity, linked with NAA, is related to the progression of disease.
Finally, Tibbo et al. (Reference Tibbo, Bernier, Hanstock, Seres, Lakusta and Purdon2013) reported a significant reduction in PCr + Cr of FEP compared with controls. Both PCr and Cr are involved in the processes related with cellular energy: thus, the authors have hypothesised that schizophrenia is associated with dysregulation in maintaining adequate energy pools. Other studies which considered PCr + Cr in their analyses (Théberge et al. Reference Théberge, Bartha, Drost, Menon, Malla, Takhar, Neufeld, Rogers, Pavlosky, Schaefer, Densmore, Al-Semaan and Williamson2002, Reference Théberge, Williamson, Aoyama, Drost, Manchanda, Malla, Northcott, Menon, Neufeld, Rajakumar, Pavlosky, Densmore, Schaefer and Williamson2007; Egerton et al. Reference Egerton, Brugger, Raffin, Barker, Lythgoe, McGuire and Stone2012; Tibbo et al. Reference Tibbo, Bernier, Hanstock, Seres, Lakusta and Purdon2013; Natsubori et al. Reference Natsubori, Inoue, Abe, Takano, Iwashiro, Aoki, Koike, Yahata, Katsura, Gonoi, Sasaki, Takao, Kasai and Yamasue2014; Dempster et al. Reference Dempster, Norman, Théberge, Densmore, Schaefer and Williamson2015) reported pressured PCr + Cr levels in FEP. It has to be noted though that Tibbo et al. (Reference Tibbo, Bernier, Hanstock, Seres, Lakusta and Purdon2013) utilised an ad-hoc MRS sequence with a long TE (240 ms) and the specific aim of quantifying mainly Cr, which could be accounted for the difference in results in respect to the other studies.
In summary, although there are some findings on increased glutamatergic levels in the ACC of FEP, they are still partially conflicting since some ¹H MRS studies found preserved concentrations. Moreover, results in FEP do not fully overlap with those in chronic schizophrenia. This could be due to the fact that metabolite levels are particularly sensitive to the characteristics of the sample, and to the phase of disease. Therefore, although glutamatergic neurotransmission may play a role in the pathophysiology of psychosis onset, the fact that findings are somewhat heterogeneous suggests that a more in-depth investigation is needed to shed light on the glutamatergic processes related to psychosis, by implementing larger longitudinal studies of FEP using ultra high field MR scanners.
Financial Support
Professor Brambilla (RF-2011-02352308) and Dr Bellani (GR-2010-2319022) were partly supported by grants from the Italian Ministry of Health. Dr Stanley was supported, in part, by the Lycaki-Young Funds from the State of Michigan.
Conflict of Interest
None.
Ethical Standard
The authors declare that no human or animal experimentation was conducted for this work.