Early life adverse events or childhood adversities (CAs) refer to a wide spectrum of stressors and detrimental experiences including physical, sexual and psychological abuses and parental neglect deeply impacting on a child's wellbeing with potential unfavourable effects on development (Browne and Winkelman, Reference Browne and Winkelman2007). Examples of early life adverse events include parental loss, maltreatment and bullying, all harming in some form the child's physical and emotional wellbeing and health (Butchart, Reference Butchart2006). According to recent studies and estimates, approximately 10% of children every year are victims of some form of maltreatment, with neglect, physical and sexual abuses being the most common (Font and Maguire-Jack, Reference Font and Maguire-Jack2020).
CAs are known to be an important risk factor for the development of several psychiatric conditions including schizophrenia and bipolar disorder (BD); recent studies suggested that CAs are significantly associated with the onset of more than 40% of all childhood psychiatric disorders and with more than 25% of adult psychiatric disorders (Green et al., Reference Green, McLaughlin, Berglund, Gruber, Sampson, Zaslavsky and Kessler2010). For example, in a recent study by Tomassi et al. (Reference Tomassi, Tosato, Mondelli, Faravelli, Lasalvia, Fioravanti, Bonetto, Fioritti, Cremonese, Parrino, Santi, Meneghelli, Torresani, Girolamo, Semrov, Pratelli, Cristofalo and Ruggeri2017) the authors found that sexual abuse was significantly associated with a diagnosis of affective psychosis and substance abuse (Tomassi et al., Reference Tomassi, Tosato, Mondelli, Faravelli, Lasalvia, Fioravanti, Bonetto, Fioritti, Cremonese, Parrino, Santi, Meneghelli, Torresani, Girolamo, Semrov, Pratelli, Cristofalo and Ruggeri2017). Other studies indicated that CAs are consistently associated with BD and that the severity of its clinical manifestations is correlated with the number and severity of CAs (Etain et al., Reference Etain, Aas, Andreassen, Lorentzen, Dieset, Gard, Kahn, Bellivier, Leboyer, Melle and Henry2013; Aas et al., Reference Aas, Henry, Andreassen, Bellivier, Melle and Etain2016). Specifically, some studies support the idea that CAs represent a risk factor for the emergence of BD, showing that the prevalence of CAs is higher in BD patients v. healthy controls (HC) for all types of CAs except for parental loss (Marangoni et al., Reference Marangoni, Hernandez and Faedda2016; Palmier-Claus et al., Reference Palmier-Claus, Berry, Bucci, Mansell and Varese2016; Perlini et al., Reference Perlini, Donisi, Rossetti, Moltrasio, Bellani and Brambilla2020). For example, in a large recent study by our group (Pedrini et al., Reference Pedrini, Ferrari, Lanfredi, Bellani, Porcelli, Caletti, Sala, Rossetti, Piccin, Dusi, Balestrieri, Perlini, Lazzaretti, Mandolini, Pigoni, Boscutti, Bonivento, Serretti, Rossi and Brambilla2021) we showed that, in BD patients, CAs are associated with worse clinical manifestations during euthymic phases and that a considerable number of patients report multiple CAs during their lifetime with neglect being more frequent v. HC (Pedrini et al., Reference Pedrini, Ferrari, Lanfredi, Bellani, Porcelli, Caletti, Sala, Rossetti, Piccin, Dusi, Balestrieri, Perlini, Lazzaretti, Mandolini, Pigoni, Boscutti, Bonivento, Serretti, Rossi and Brambilla2021). Moreover, the number and severity of CAs has been shown to influence clinical factors such as age of onset, risk for suicide, frequency of mood episodes and response to treatment (Aas et al., Reference Aas, Henry, Andreassen, Bellivier, Melle and Etain2016; Nemeroff, Reference Nemeroff2016).
Several biopsychosocial models have been proposed for the integration of genetic vulnerabilities and socio-environmental factors in psychotic and bipolar disorders including the multiple-hit models of susceptibility (Aas et al., Reference Aas, Henry, Andreassen, Bellivier, Melle and Etain2016). Briefly, these models take into account the wide heterogeneity of individuals suffering from CAs and integrate genetic susceptibilities, environmental factors and their complex interactions aiming at understanding why only some of them develop psychiatric conditions, such as BD. According to these models, different neurodevelopmental trajectories are determined by the interplay between all these variables ultimately giving rise to BD only in a relatively small portion of individuals. For example, in a recent work by Jaworska-Andryszewska and Rybakowski, the authors suggested that CAs can affect specific regions of the central nervous system (e.g. hippocampus and amygdala) associated with the development of mood disorders (Jaworska-Andryszewska and Rybakowski, Reference Jaworska-Andryszewska and Rybakowski2019). This is due to the interaction of environmental and genetic factors, the most important being the serotonin transporter gene and the FKBP5 gene. However, the exact mechanisms and trajectories of these models are still unclear including their neurobiological correlates.
Increasing evidence from neuroimaging studies suggest that CAs are associated with multiple brain alterations in healthy individuals mainly localised in the prefrontal cortex, hippocampus, amygdala, corpus callosum (CC) and other white matter (WM) structures (McCrory et al., Reference McCrory, De Brito and Viding2011; Lim et al., Reference Lim, Radua and Rubia2014; Teicher et al., Reference Teicher, Samson, Anderson and Ohashi2016). Similarly, early life traumas have been suggested to alter WM structures, grey matter (GM) volumes and functional connectivity of several brain regions in patients suffering from psychosis (Cancel et al., Reference Cancel, Dallel, Zine, El-Hage and Fakra2019). However, little is known about the possible effects of CAs in the BD brain. Considering the potential impact of CAs on brain development and their relevance in the development and characterisation of many clinical features of BD, it is crucial to better elucidate on the neurobiological correlates of CAs in BD. Therefore, this paper reviewed existing literature discussing the association between CAs and brain alterations in BD patients.
The data search was conducted on the PubMed, Scopus and Google Scholar databases. The following query was used for the search: (‘bipolar disorder’) AND (‘imaging’ OR ‘neuroimaging’ OR ‘magnetic resonance imaging’ OR ‘magnetic resonance’ OR ‘electroencephalography’ OR ‘Positron Emission Tomography’ OR ‘diffusion tensor imaging’ OR ‘brain’ OR ‘grey matter’ OR ‘gray matter’ OR ‘white matter’) AND (‘childhood abuse’ OR ‘childhood maltreatment’ OR ‘childhood trauma’ OR ‘early life adverse events’ OR ‘early life stress’ OR ‘adverse events’ OR ‘neglect’). Preclinical studies and case-report were excluded. A flow diagram illustrating the studies selection process is presented in Fig. 1. The literature search retrieved 136 records. After title and abstract screening, 121 articles were excluded because they clearly did not meet the inclusion criteria. The remaining 14 studies were included in this review after a full-text review. Sample characteristics and neuroimaging findings from each study are shown in Table 1. The data search was conducted on September 2021.
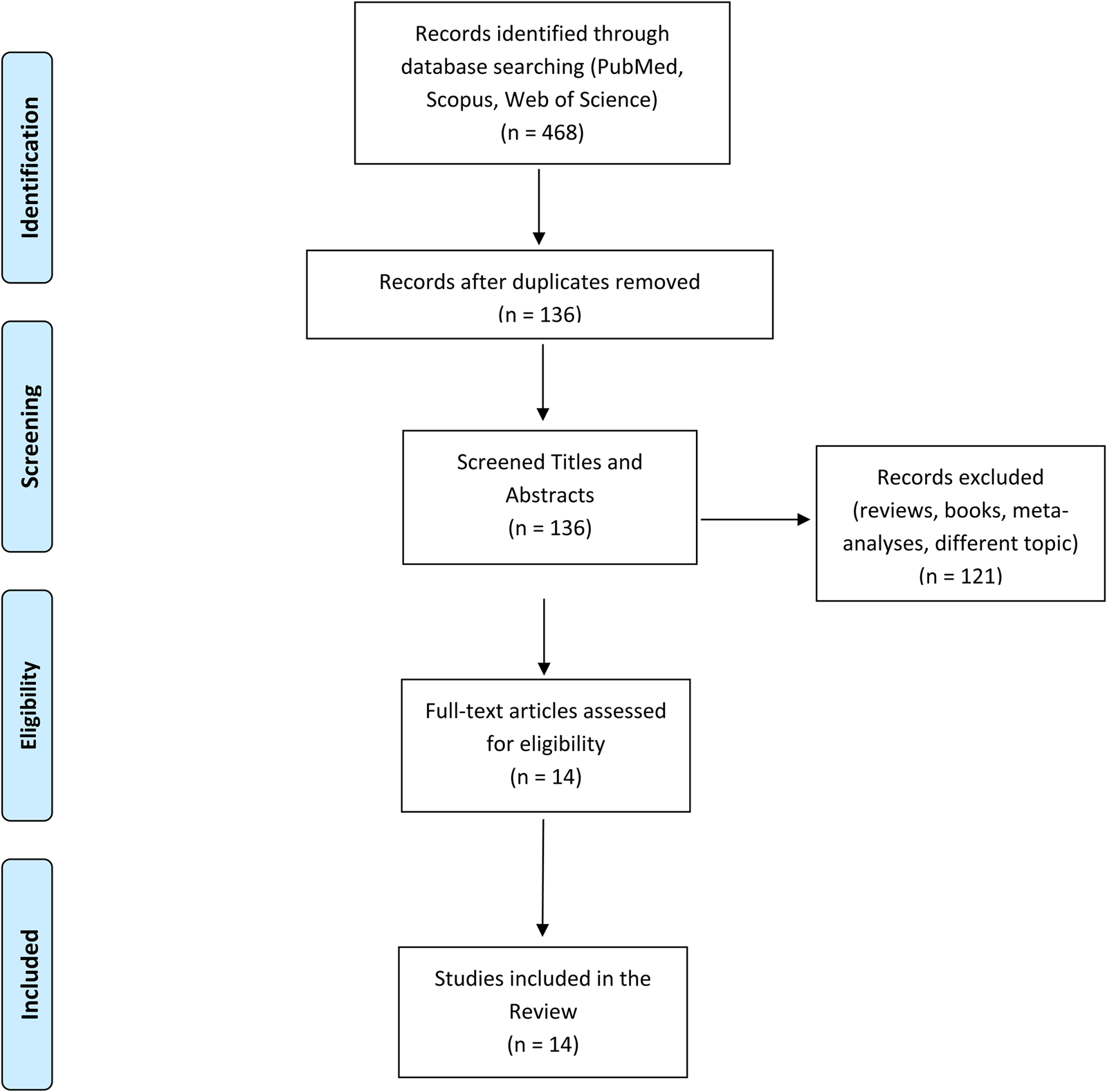
Fig. 1. A graphic representation of our search query including the exact number of studies found and excluded.
Table 1. Studies investigating the neurobiological correlates of early adversities in bipolar disorder

BD, bipolar disorder; BD-I, bipolar disorder type 1; BDNF, brain-derived neurotrophic factor; BOLD, blood oxygenation level-dependent; CC, corpus callosum; CTQ, Childhood Trauma Questionnaire; GM, grey matter; HC, healthy controls; OFC, orbitofrontal cortex; PFC, prefrontal cortex; RFQ, Risky Families Questionnaire; SCZ, schizophrenia; WM, white matter.
Of all the included studies, 85% (12 out of 14) explored the neuro-structural correlates of CAs in BD (i.e. GM volumes, WM structural integrity) through magnetic resonance imaging and diffusion tensor imaging. Only a handful of studies (two out 14) explored the functional correlates of CAs in BD. Finally, two studies explored the association between brain-derived neurotrophic factor levels and CAs in BD patients showing that patients with a history of CA are marked by lower levels (Aas et al., Reference Aas, Haukvik, Djurovic, Tesli, Athanasiu, Bjella, Hansson, Cattaneo, Agartz, Andreassen and Melle2014, Reference Aas, Dieset, Mørch, Steen, Hope, Reponen, Laskemoen, Ueland, Aukrust, Melle, Agartz and Andreassen2019). The Childhood Trauma Questionnaire (CTQ) was used to assess and quantify CAs in the sample in 13 out of the 14 studies (92%) (Bernstein et al., Reference Bernstein, Fink, Handelsman, Foote, Lovejoy, Wenzel, Sapareto and Ruggiero1994). Briefly, the CTQ is a retrospective self-report questionnaire assessing and quantifying CAs including neglect, physical, emotional and sexual abuses. It is composed of 70 items and responses are quantified on a five-point Likert scale according to the frequency with which CAs occurred (1 = ‘never true’ and 5 = ‘very often true’).
Studies investigating the association between CAs and neuro-structural alterations showed mixed findings. Higher levels of CAs correlated with the reductions of the hippocampal volumes in BD patients (Aas et al., Reference Aas, Haukvik, Djurovic, Bergmann, Athanasiu, Tesli, Hellvin, Steen, Agartz, Lorentzen, Sundet, Andreassen and Melle2013, Reference Aas, Haukvik, Djurovic, Tesli, Athanasiu, Bjella, Hansson, Cattaneo, Agartz, Andreassen and Melle2014; Janiri et al., Reference Janiri, Sani, Rossi, Piras, Iorio, Banaj, Giuseppin, Spinazzola, Maggiora, Ambrosi, Simonetti and Spalletta2017, Reference Janiri, Sani, De Rossi, Piras, Banaj, Ciullo, Simonetti, Arciniegas and Spalletta2019) with only one study showing larger hippocampal volumes (Janiri et al., Reference Janiri, Sani, Rossi, Piras, Iorio, Banaj, Giuseppin, Spinazzola, Maggiora, Ambrosi, Simonetti and Spalletta2017). Specifically, BD patients with CAs were compared with other patients without a history of CAs (Janiri et al., Reference Janiri, Sani, Rossi, Piras, Iorio, Banaj, Giuseppin, Spinazzola, Maggiora, Ambrosi, Simonetti and Spalletta2017) or with HC (Janiri et al., Reference Janiri, Sani, De Rossi, Piras, Banaj, Ciullo, Simonetti, Arciniegas and Spalletta2019) or divided into two groups according to specific genetic vulnerabilities (Aas et al., Reference Aas, Haukvik, Djurovic, Bergmann, Athanasiu, Tesli, Hellvin, Steen, Agartz, Lorentzen, Sundet, Andreassen and Melle2013, Reference Aas, Haukvik, Djurovic, Tesli, Athanasiu, Bjella, Hansson, Cattaneo, Agartz, Andreassen and Melle2014). Other structural studies investigating the possible GM correlates of CAs in BD patients showed volume reductions in the thalamus (Duarte et al., Reference Duarte, Neves, Albuquerque, de Souza-Duran, Busatto and Corrêa2016; Poletti et al., Reference Poletti, Vai, Smeraldi, Cavallaro, Colombo and Benedetti2016), alterations in the amygdala (Souza-Queiroz et al., Reference Souza-Queiroz, Boisgontier, Etain, Poupon, Duclap, d'Albis, Daban, Hamdani, Le Corvoisier, Delavest, Bellivier, Guevara, Leboyer, Henry and Houenou2016; Janiri et al., Reference Janiri, Sani, Rossi, Piras, Iorio, Banaj, Giuseppin, Spinazzola, Maggiora, Ambrosi, Simonetti and Spalletta2017) and in several frontal regions including the pre-frontal, orbito-frontal, para-central cortices and the middle-frontal gyrus (Duarte et al., Reference Duarte, Neves, Albuquerque, de Souza-Duran, Busatto and Corrêa2016; Poletti et al., Reference Poletti, Vai, Smeraldi, Cavallaro, Colombo and Benedetti2016; Song et al., Reference Song, Chon, Ryu, Yu, Lee, Lee, Lee, Lee and Park2020; Begemann et al., Reference Begemann, Schutte, van Dellen, Abramovic, Boks, van Haren, Mandl, Vinkers, Bohlken and Sommer2021). As shown in Table 1, these alterations seem to indicate that BD patients with a history of CAs are characterised by volume reductions in all these regions when compared with HC or similar group without a history of CAs. Lastly, studies investigating WM integrity showed an association between a history of CAs in BD and a reduction of volumes and fractional anisotropy (FA) in the CC, uncinate fasciculus and corona radiata when compared with HC or BD patients without CAs (Bücker et al., Reference Bücker, Muralidharan, Torres, Su, Kozicky, Silveira, Bond, Honer, Kauer-Sant'anna, Lam and Yatham2014; Souza-Queiroz et al., Reference Souza-Queiroz, Boisgontier, Etain, Poupon, Duclap, d'Albis, Daban, Hamdani, Le Corvoisier, Delavest, Bellivier, Guevara, Leboyer, Henry and Houenou2016; Stevelink et al., Reference Stevelink, Abramovic, Verkooijen, Begemann, Sommer, Boks, Mandl, van Haren and Vinkers2018).
Functional studies exploring the association between CAs and neuro-functional alterations in BD are scarce and show mixed findings employing both at-rest and task-related designs. For example, Souza-Queiroz et al. showed that, when compared with HC, BD patients with a history of CA are marked by lower pre-fronto-limbic connectivity at rest (Souza-Queiroz et al., Reference Souza-Queiroz, Boisgontier, Etain, Poupon, Duclap, d'Albis, Daban, Hamdani, Le Corvoisier, Delavest, Bellivier, Guevara, Leboyer, Henry and Houenou2016). This finding was partially replicated by another study by Hsieh et al. (Reference Hsieh, Wu, Tseng, Wei, Huang, Chang, Yang and Chen2021) showing the presence of lower connectivity between left-caudate and the fronto-parietal network, right supra-marginal gyrus, left inferior parietal lobule, right middle frontal gyrus and right superior parietal lobule in BD patients with a history of physical neglect. Lastly, only a single study employed a task-related design showing that BD patients with a history of childhood trauma present stronger activations in the right angular, supra-marginal, middle temporal gyri and lateral occipital cortex during an emotion recognition task (Aas et al., Reference Aas, Kauppi, Brandt, Tesli, Kaufmann, Steen, Agartz, Westlye, Andreassen and Melle2017).
Overall, our review suggests that in BD patients, CAs are associated with GM alterations of the hippocampus, thalamus and across prefrontal regions (i.e. dorsolateral prefrontal, orbitofrontal cortices and precentral, middle frontal gyri). These alterations are expressed as volume reductions with only a paucity of studies showing opposite trends (Janiri et al., Reference Janiri, Sani, Rossi, Piras, Iorio, Banaj, Giuseppin, Spinazzola, Maggiora, Ambrosi, Simonetti and Spalletta2017). Moreover, patients with a history of CAs are also distinguished by alterations of the CC mainly expressed as volume or FA reductions. These results are not entirely surprising; in fact, the hippocampus has been previously shown to be altered in psychotic patients with a history of CAs, especially in the early phases of the condition (Hoy et al., Reference Hoy, Barrett, Shannon, Campbell, Watson, Rushe, Shevlin, Bai, Cooper and Mulholland2012). Similar findings have been previously associated with cognitive dysfunctions in both BD and schizophrenia in several key cognitive domains including psychomotor speed, memory and executive functioning (Knöchel et al., Reference Knöchel, Stäblein, Storchak, Reinke, Jurcoane, Prvulovic, Linden, van de Ven, Ghinea, Wenzler, Alves, Matura, Kröger and Oertel-Knöchel2014, Reference Knöchel, Stäblein, Prvulovic, Ghinea, Wenzler, Pantel, Alves, Linden, Harrison, Carvalho, Reif and Oertel-Knöchel2016, Reference Knöchel, Kniep, Cooper, Stäblein, Wenzler, Sarlon, Prvulovic, Linden, Bahn, Stocki, Ozcan, Alves, Carvalho, Reif and Oertel-Knöchel2017). Alterations of the hippocampus – the most consistent finding of our review – could be due to the fact that the hippocampus is part of the hypothalamus–pituitary–adrenal axis known to be influenced by stressful events and possibly leading to volume alterations (Heim et al., Reference Heim, Newport, Mletzko, Miller and Nemeroff2008). Notably, these alterations are known also for other psychiatric disorders such as post-traumatic stress disorder, schizophrenia and depression (de Kloet et al., Reference de Kloet, Vermetten, Geuze, Kavelaars, Heijnen and Westenberg2006; Wingenfeld and Wolf, Reference Wingenfeld and Wolf2011; Belvederi Murri et al., Reference Belvederi Murri, Pariante, Mondelli, Masotti, Atti, Mellacqua, Antonioli, Ghio, Menchetti, Zanetidou, Innamorati and Amore2014). Moreover, microglial activations – an indicator of neuronal damage – have been found in both thalamus and hippocampus in rodents exposed to stressors suggesting a specific effect of stress on mammals' brains (Sugama et al., Reference Sugama, Fujita, Hashimoto and Conti2007). Likewise, alterations of the thalamus, prefrontal regions and CC have also been suggested to be present in schizophrenic patients with a history of CAs, possibly indicating a transdiagnostic effect, linking both affective and non-affective conditions (Cancel et al., Reference Cancel, Dallel, Zine, El-Hage and Fakra2019). Notably, the thalamus is connected through sensory fibres with the amygdala playing an important role in filtering sensory information and regulating emotional responses (Herrero et al., Reference Herrero, Barcia and Navarro2002).
Several limitations should be considered when interpreting the results of our review. First, studies investigating the association between CAs and neurobiological variations from the norm are cross-sectional and retrospective. Moreover, the magnitude of the observed effects is unclear as none of the studies reported the effect size or the confidence intervals of the results. Lastly, only a handful of studies differentiated between different types of CAs or compared patients' group with and without a history of CAs. Therefore, future studies are warranted taking into account these limitations informing future diagnostic systems and interventions about the exact neurobiological firms of CAs in the BD brain.
Data
The data that support the findings of this study (search query) are available on request from the corresponding author.
Financial support
CP was partially supported by grant from the Italian Ministry of Health (GR-2016-02361283).
Conflict of interest
None.




