- ICU
intensive care unit
- MCT, TAG
containing predominantly medium-chain fatty acids
This review presents the rationale for the inclusion of marine n-3 fatty acids, in the form of fish oil, in artificial nutrition regimens for use in various patient groups, including both parenteral and enteral applications. The review goes on to describe and interpret studies using parenteral or enteral fish oil in various patient groups with a focus on immune, inflammatory and clinical outcomes. The material described is largely based upon that presented in previous review articles of this subject(Reference Calder1–Reference Calder3), but here this information is updated with respect to additional studies of relevance(Reference Liang, Wang and Ye4), new meta-analyses(Reference Pontes-Arruda, Demichele, Seth and Singer5) and newly published guidelines for parenteral nutrition(Reference Braga, Ljungqvist and Soeters6, Reference Singer, Berger and Van den Berghe7).
Fatty acids
Fatty acids are hydrocarbon chains with a carboxyl group at one end and a methyl group at the other(Reference Calder, Burdge, Nicolaou and Kafatos8). The carboxyl group is reactive and readily forms ester links with alcohol groups, for example those on glycerol or cholesterol, in turn forming acylglycerols (e.g. TAG and phospholipids) and cholestryl esters. Fatty acid chain lengths vary from two to 30 or more and the chain may contain double bonds. Fatty acids containing double bonds in the hydrocarbon chain are referred to as unsaturated fatty acids; a fatty acid containing one double bond is called a monounsaturated fatty acid, while one containing two or more double bonds is called a PUFA. Fatty acids have common names (Table 1) and systematic names. They are also referred to by a shorthand nomenclature that denotes the number of carbon atoms in the chain, the number of double bonds and the position of the first double bond relative to the methyl (n) carbon (Table 1). n-3 and n-6 fatty acids are so-called because the first double bond is on carbon number 3 or 6, respectively, counting the methyl carbon as carbon number 1. The simplest n-6 fatty acid is linoleic acid (18:2n-6) and the simplest n-3 fatty acid is α-linolenic acid (18:3n-3). Linoleic and α-linolenic acids cannot be synthesised in animals, including human subjects. They are the classical essential fatty acids. In contrast, saturated and monounsaturated fatty acids can be synthesised de novo in human subjects(Reference Calder2).
Table 1. Common names, shorthand nomenclature and typical sources of fatty acids used in artificial nutrition. Modified from Calder(Reference Calder3)
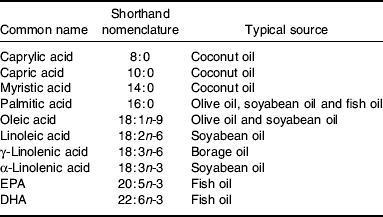
Although mammalian cells cannot synthesise linoleic and α-linolenic acids, they can metabolise them by further desaturation and elongation. Linoleic acid can be converted to γ-linolenic acid (18:3n-6), then to dihomo-γ-linolenic acid (20:3n-6) and then to arachidonic acid (20:4n-6). Using the same series of enzymes, α-linolenic acid is converted to EPA (20:5n-3). A complex pathway for further conversion of EPA to DHA (22:6n-3) exists(Reference Calder, Burdge, Nicolaou and Kafatos8–Reference Sprecher10). Fatty acids that are important in artificial nutrition and their typical sources for this purpose are listed in Table 1.
Desirable properties for lipids to be used in artificial nutrition
Lipids used in artificial nutrition should provide:
a source of energy as an alternative to glucose;
building blocks, since patients requiring artificial nutrition will typically be undergoing processes involving cell replication and tissue repair;
essential fatty acids in order to avoid deficiency symptoms;
a ‘good’ fatty acid balance, although the precise definition of this balance is still lacking;
fatty acids with desirable biological activities; it is now recognised that fatty acids can affect cell membrane properties, cell signalling, gene expression and the production of bioactive mediators(Reference Calder, Burdge, Nicolaou and Kafatos8, Reference Yaqoob and Calder11, Reference Calder12), and it would be desirable if the fatty acids provided as a component of artificial nutrition, at worst, did not exacerbate inappropriate cellular responses and, at best, modulated these in a manner that would improve patient outcome.
Use of fish oil in parenteral nutrition in surgical and critically ill patients
Rationale for the use of fish oil in parenteral nutrition
Lipids were first introduced into parenteral nutrition formulas in the 1960s in order to provide a more balanced supply of energy, along with glucose(Reference Edgren and Wrtelind13–Reference Wretlind15). The lipid typically used in parenteral nutrition is soyabean oil, in which linoleic acid comprises about 50% of fatty acids present. A meta-analysis of total parenteral nutrition suggested that inclusion of lipids might be detrimental (P=0·09 for lipids v. no lipids)(Reference Heyland, MacDonald, Keefe and Drover16), at least in very ill patients; most of the studies included in the meta-analysis used soyabean oil-based lipid emulsions. A more recent study in patients following major gastrointestinal surgery identified that the amount of n-6 PUFA (i.e. linoleic acid) infused was one of two predictors of the length of hospital stay (increased by 1·6 d/100 g of n-6 PUFA infused), the other being the delay in the onset of initiating nutritional support(Reference Koch and Heller17). A number of in vitro experiments have shown that soyabean oil-based lipid emulsions can exert immunosuppressive effects (see Calder et al.(Reference Calder, Sherrington, Askanazi and Newsholme18) for references), which would clearly be detrimental in patients at risk of infection and sepsis. Clinical trials with soyabean oil-based lipid emulsions provide conflicting evidence, some showing selective immunosuppressive effects(Reference Monson, Sedman and Ramsden19–Reference Furukawa, Yamamori and Takagi21), perhaps linked to poorer patient outcomes(Reference Battistella, Widergren and Anderson20). However, other studies do not show such effects on the immune system(Reference Dionigi, Dionigi and Prati22–Reference Sedman, Somers and Ramsden24) or on clinical outcomes(Reference Lenssen, Bruemmer and Bowden25). These studies have been described and discussed previously(Reference Calder1, Reference Calder3). Despite the inconsistencies of the outcomes of such studies, a view has developed that the use of lipid emulsions based solely upon soyabean oil may not be optimal or may even be harmful. The concern about potential harm, based mainly on the idea that n-6 PUFA might be ‘pro-inflammatory, immunosuppressive and pro-coagulatory’, has led to the development of alternative lipid emulsions for parenteral applications. Two alternative philosophies towards reducing the amount of linoleic acid have been adopted. The first has been to simply dilute soyabean oil with another oil that is fairly inert. Examples of this strategy include the use of so-called medium-chain TAG (MCT; TAG containing predominantly medium-chain fatty acids) and olive oil. This approach is not discussed further here, but has been described in detail elsewhere(Reference Ulrich and McCarthy Pastores26–Reference Sala-Vila, Barbosa and Calder28). The second approach has been to partially replace soyabean oil with another oil that is believed to exert benefits in its own right. An example of this strategy is the use of fish oil(Reference Furst and Kuhn29–Reference Grimm32).
Fish oil contains the very long-chain n-3 PUFA EPA and DHA. There is strong evidence for health benefits of these fatty acids, especially with regard to CVD(Reference Calder33–Reference Studer, Briel and Leimenstoll36). They act to modify tissue and blood lipid metabolism, blood lipid concentrations, blood coagulation, immune function, inflammation and endothelial function(Reference Wang, Harris and Chung37–Reference Calder41). EPA and DHA are readily incorporated into cells and tissues and act to modify membrane properties, eicosanoid profiles, signal transduction processes and gene expression(Reference Yaqoob and Calder11, Reference Calder12). Through these mechanisms they result in improved cell and tissue function. Thus, using fish oil to partly replace soyabean oil in parenteral nutrition offers the possibility to both decrease the amount of linoleic acid present and increase the amount of biologically active n-3 PUFA.
Animal feeding studies have demonstrated that fish oil decreases the production of inflammatory eicosanoids and cytokines in endotoxaemia(Reference Utsunomiya, Chavali, Zhong and Forse42–Reference Sadeghi, Wallace and Calder44) or sepsis(Reference Barton, Wells and Carlson45–Reference Lanza-Jacoby, Flynn and Miller47) and that this is associated with a decreased metabolic response(Reference Mulrooney and Grimble48–Reference Teo, Selleck and Wan51), improved organ function(Reference Sane, Baba and Kusano43, Reference Sane, Baba and Kusano52–Reference Mancuso, Whelan and DeMichele57) and improved survival(Reference Barton, Wells and Carlson45–Reference Barton, Wells and Carlson47, Reference Barton, Wells and Carlson58–Reference Johnson, Griswold and Muakkassa60).
Three lipid emulsions that include fish oil as a component are currently available. Omegaven® (Fresenius Kabi, Bad Homberg, Germany) is a pure fish oil emulsion (100 g lipid/l) that will typically contain about 3 g EPA plus DHA/100 ml. It is recommended that Omegaven is used in combination with other emulsions (e.g. those based on soyabean oil) such that Omegaven contributes 10–20% of infused emulsion. Lipoplus® (also known as Lipidem®; B. Braun, Melsungen, Germany) is an emulsion (200 g lipid/l) with the lipid being a mix of 50% MCT, 40% soyabean oil and 10% fish oil. Each 100 ml of Lipoplus will typically contain about 0·6 g EPA plus DHA. SMOFLipid® (Fresenius Kabi, Bad Homberg, Germany) is an emulsion (200 g lipid/l) with the lipid being a mix of 30% MCT, 30% soyabean oil, 25% olive oil and 15% fish oil. Each 100 ml of SMOFLipid® will typically contain about 1 g EPA plus DHA.
Studies of parenteral fish oil in surgical patients
Intravenous infusion of lipid emulsions containing fish oil into patients following gastrointestinal surgery altered the fatty acid composition of plasma(Reference Morlion, Torwesten and Lessire61–Reference Senkal, Geier and Hannemann64), platelet(Reference Roulet, Frascarolo, Pilet and Chapuis65) and erythrocyte(Reference Senkal, Geier and Hannemann64) phospholipids: typically EPA content was increased. Intravenous infusion of a lipid emulsion containing fish oil into patients for 5 d following gastrointestinal surgery also altered the fatty acid composition of leucocytes: EPA content was increased 2·5-fold(Reference Wachtler, Konig and Senkal66). This would be expected to impact on the profile of eicosanoids produced from arachidonic acid and EPA. Indeed, several studies have demonstrated that intravenous infusion of lipid emulsions containing fish oil into patients who had undergone major gastrointestinal surgery results in lower production of arachidonic acid-derived eicosanoids and higher production of EPA-derived eicosanoids by blood leucocytes stimulated ex vivo (Reference Morlion, Torwesten and Lessire61, Reference Grimm, Mertes and Goeters62, Reference Wachtler, Konig and Senkal66, Reference Koller, Senkal and Kemen67). Plasma TNF-α concentrations were lower at day 6 post-surgery, while plasma IL-6 concentrations were lower at day 10 post-surgery in patients who had undergone major gastrointestinal surgery and then received a mix of MCT, soyabean oil and fish oil (50:30:20 by vol.; this was a prototype version of Lipoplus) for 5 d post-surgery compared with those who received an MCT–soyabean oil mix(Reference Wachtler, Konig and Senkal66). The study did not report clinical outcomes. Another study infused Omegaven, providing 10 g fish oil/d, on the day before abdominal surgery and on days 1–5 following abdominal surgery(Reference Weiss, Meyer and Matthies68). On days 4 and 5 the patients also received standard total parenteral nutrition, which included 50 g fat/d as soyabean oil. TNF-α production by endotoxin-stimulated whole blood tended to be lower at post-operative day 5 in the fish oil group, but this was not significant. Serum IL-6 concentrations were significantly lower at days 0, 1 and 3 in the fish oil group than in controls. Monocyte expression of human leucocyte antigen-DR, an indication of ability to present antigen and so to mount an immune response, was preserved in the fish oil group, but declined at post-surgery days 3 and 5 in the control group. No differences in infection rates or mortality were observed. However, post-operative stay in intensive care tended to be shorter in the fish oil group (4·1 v. 9·1 d in the control group) as did total hospital stay (17·8 v. 23·5 d). Post-operative stay on medical wards was significantly shorter in the fish oil group (P<0·05). Another study compared the effects of lipid-free total parenteral nutrition or parenteral nutrition including soyabean oil or a mix of 83% soyabean oil and 17% fish oil from Omegaven for 5 d after large bowel surgery(Reference Schauder, Rohn and Schafer69). There were no differences between the groups with respect to the numbers of circulating lymphocytes, B lymphocytes, helper T lymphocytes, cytotoxic T lymphocytes or natural killer cells before surgery or at days 3 and 6 post-surgery, although these were affected by surgery itself. There were no differences between groups with respect to T lymphocyte proliferation, but IL-2 production was increased in the fish oil group and the post-surgery decline in interferon-γ production was prevented by fish oil. Liang et al.(Reference Liang, Wang and Ye4) compared soyabean oil with a mixture of soyabean oil and Omegaven (5:1, v/v) over 7 d in patients who had undergone radical colorectal cancer resection. The decline in serum IL-6 concentration between post-operative day 1 and day 8 was greater in the group receiving fish oil, while the increase in the ratio of CD4+ to CD8+ cells in the bloodstream, believed to be a marker of the cell-mediated immune response, was greater in that group. Length of hospital stay tended to be shorter in the fish oil group (17·5 v. 19·6 d in the control group) and infectious complications and mortality were not different between groups. Wichmann et al.(Reference Wichmann, Thul and Czarnetzki63) reported length of hospital stay in post-gastrointestinal surgery patients receiving soyabean oil or Lipoplus: length of stay was significantly shorter (P=0·006) in patients receiving fish oil (17·2 d) than in the control group (21·9 d). In another study in post-surgical patients, SMOFLipid for 6 d resulted in significantly shorter hospital stay (13·4 v. 20·4 d; P<0·05) than soyabean oil(Reference Grimm, Mertes and Goeters62). Taken together, these studies indicate that inclusion of fish oil in parenteral nutrition regimens for gastrointestinal surgical patients modulates the generation of inflammatory eicosanoids(Reference Morlion, Torwesten and Lessire61, Reference Grimm, Mertes and Goeters62, Reference Wachtler, Konig and Senkal66, Reference Koller, Senkal and Kemen67) and cytokines(Reference Liang, Wang and Ye4, Reference Wachtler, Konig and Senkal66, Reference Weiss, Meyer and Matthies68) and may help to counter the surgery-induced decline in antigen presenting cell activity(Reference Weiss, Meyer and Matthies68) and T lymphocyte cytokine production(Reference Schauder, Rohn and Schafer69). Importantly, these studies do not reveal any deleterious effects of fish oil infusion in these patients. Furthermore, studies that have examined the hard endpoint of length of hospital stay suggest a real clinical benefit from fish oil infusion in these patients(Reference Grimm, Mertes and Goeters62, Reference Wichmann, Thul and Czarnetzki63, Reference Weiss, Meyer and Matthies68). Another report from a cohort of patients receiving parenteral nutrition post-surgery also indicates benefit from the inclusion of fish oil in the regimen(Reference Tsekos, Reuter, Stehle and Boeden70). There were no differences between the control group (MCT–soyabean oil) and the patients receiving fish oil (a mix of Omegaven with a 50:50 MCT–soyabean oil mix, where a maximum of one-third of the mix was as fish oil) with respect to the proportion of patients who developed wound infections (6% in the fish oil group v. 11% in the control group) or who died (12% v. 15%), or in the length of hospital stay (25 v. 29 d). However, the proportion of patients in the fish oil group who were readmitted to intensive care (5%) was significantly lower (P<0·05) than in the control group (17%). A group of patients also received the fish oil-containing emulsion for 2 d preoperatively. Here there were a number of very significant benefits. This group showed a significantly decreased need for mechanical ventilation (17% v. 31% in the control group; P<0·05), a significantly shorter length of hospital stay (22 v. 29 d; P<0·05), significantly less need for readmission to intensive care (5% v. 17%; P<0·05) and significantly lower mortality (3% v. 15%; P<0·05)(Reference Tsekos, Reuter, Stehle and Boeden70). Another study revealed that intravenous infusion of a lipid emulsion containing 80% soyabean oil and 20% Omegaven into patients for 5 d following major gastrointestinal surgery accelerated normalisation of liver and pancreatic function compared with soyabean oil alone(Reference Heller, Rossel and Gottschlich71). Overall, there was no difference between the groups with respect to length of stay in the intensive care unit (ICU) or in hospital. However, in a subgroup of patients at risk of sepsis, there was a reduced ICU stay in patients receiving fish oil (4·0 v. 5·3 d in the control group; P=0·01)(Reference Heller, Rossel and Gottschlich71). In a recently published study, a mixed group of over 650 patients including about 230 post-surgical patients received parenteral nutrition including fish oil (Omegaven) for at least 3 d (mean 8·7 d); there was a significantly lower rate of infections (P<0·0005), fewer complications (P<0·005) and a shorter length of hospital stay (P=0·05) in post-surgery patients receiving fish oil compared with those receiving the control emulsion(Reference Koch and Heller17). These authors identified that infusion of about 0·15 g fish oil/kg per d decreased mean ICU stay from 8·7 to 5·3 d and hospital stay from 27·4 to 25·5 d. Thus, findings available from published studies in gastrointestinal surgical patients fairly clearly demonstrate clinical benefit from the inclusion of very long-chain n-3 PUFA in the form of fish oil in parenteral nutrition regimens(Reference Koch and Heller17, Reference Grimm, Mertes and Goeters62, Reference Wichmann, Thul and Czarnetzki63, Reference Weiss, Meyer and Matthies68, Reference Tsekos, Reuter, Stehle and Boeden70, Reference Heller, Rossel and Gottschlich71). However, the study of Tsekos et al.(Reference Tsekos, Reuter, Stehle and Boeden70) also demonstrates a greater benefit if these fatty acids are additionally provided pre-surgery, which, of course, is only possible in elective surgery. The greater benefit of pre-operative infusion of long-chain n-3 PUFA most likely relates to better incorporation of the fatty acids into leucocytes and other tissues.
Recently, a study using MCT–soyabean oil or Lipoplus in ICU patients having undergone abdominal aorta aneurysm repair surgery was published(Reference Berger, Tappy and Revelly72). There were no differences in glucose metabolism or in inflammatory markers. Clinical outcomes were not affected either, but there was a trend towards shorter ICU stay (1·6 v. 2·3 d) and shorter hospital stay (9·9 v. 11·3 d).
Thus, all three available fish oil-containing lipid emulsions have been used in adult post-surgery (mainly gastrointestinal) patients. No adverse effects of the use of fish oil have been reported, indicating that it is safe to use in such patients. The use of fish oil is associated with altered patterns of inflammatory eicosanoids and cytokines in post-gastrointestinal surgery patients, and immune function may be better maintained by fish oil in these patients. Two studies reported that the use of fish oil is associated with a trend towards reduced length of ICU stay and three studies reported that fish oil significantly reduced length of hospital stay (three more studies reported a trend to reduced length of hospital stay). Lack of significance in studies that reported favourable trends may be due to the small sample size of those studies. Perioperative administration of fish oil may be superior to post-operative. Taken together the studies in post-surgery patients present a fairly consistent and positive view of the efficacy of intravenous fish oil administration post-surgery. However, in these studies patients who would not normally require parenteral nutrition have frequently been included. Furthermore, the lengths of ICU and hospital stay reported in both control and fish oil groups are frequently much longer than typically seen in many clinical settings. Thus, although the data presently available are highly supportive of the inclusion of fish oil, translation of the findings to the real clinical situation requires further studies designed to mimic current clinical practice; clearly such studies need to be properly designed and adequately powered.
Studies of parenteral fish oil in critically ill patients
Septic patients who were intolerant of enteral nutrition received a standard soyabean oil-based emulsion or an emulsion containing fish oil (Omegaven) for 5(Reference Mayer, Fegbeutel and Hattar73) or 10(Reference Mayer, Gokorsch and Fegbeutel74) d. Blood leucocyte counts and serum C-reactive protein concentration tended to be lower, and production of leukotriene B5 by stimulated neutrophils was significantly higher in patients receiving fish oil(Reference Mayer, Fegbeutel and Hattar73). Production of TNF-α, IL-1β, IL-6, IL-8 and IL-10 by endotoxin-stimulated mononuclear cells did not increase during infusion of the fish oil-containing emulsion, whereas production of the four pro-inflammatory cytokines was markedly elevated during the first 2 d of soyabean oil infusion(Reference Mayer, Gokorsch and Fegbeutel74). These studies establish that infusion of long-chain n-3 PUFA into patients with sepsis can modulate inflammatory mediator production and related inflammatory processes. It has been demonstrated that this might be associated with clinical improvements. Heller et al.(Reference Heller, Rössler and Litz75) included patients with abdominal sepsis, multiple trauma and severe head injury in their study of parenteral n-3 PUFA (in the form of Omegaven) infusion. They found a significantly lower rate of infection and shorter lengths of ICU and hospital stay in those patients receiving more than 0·05 g fish oil/kg per d than in those receiving less than this. Mortality was significantly decreased in those patients who received more than 0·1 g fish oil/kg per d. The survival advantage was greater in some patient groups than others (severe head injury>multiple trauma>abdominal sepsis>non-abdominal sepsis>post-surgery), but small numbers of patients in some groups make the interpretation of these data difficult. Furthermore, this study was not controlled or blinded. Nevertheless, these recent data are strongly suggestive of genuine clinical benefit from the inclusion of long-chain n-3 PUFA in parenteral nutrition regimens given to critically ill patients. This conclusion is in part supported by a study in patients with severe acute pancreatitis(Reference Wang, Li, Li and Li76). The patients received soyabean oil or a mixture of soyabean oil and Omegaven for 5 d. Although there were no differences between the groups with regard to inflammatory markers, number of infections, or lengths of ICU (27·5 d in the control group v. 21·4 d in the fish oil group) and hospital stay, there was better gas exchange (P<0·05) and a reduced requirement for continuous renal replacement therapy (P<0·05) in those patients receiving fish oil. In contrast to the generally positive findings from the above studies, Friesecke et al.(Reference Friesecke, Lotze and Köhler77) reported no differences between MCT–soyabean oil and MCT–soyabean oil–Omegaven given over 7 d in medical ICU patients in several outcomes, including immune markers, inflammatory markers, bleeding, ventilation requirement, number of infections, length of ICU stay or mortality.
Thus, of the three available fish oil-containing lipid emulsions, only Omegaven has been used in critically ill adults. No adverse effects of the use of fish oil have been reported in these studies, indicating that it is safe to use in such patients. The influence of fish oil on inflammatory processes and on immune function in critically ill patients is not yet clear. Similarly, the impact of fish oil on clinical endpoints like infections, length of ICU and hospital stay, and mortality is not clear, since there are too few studies and those that are available(Reference Heller, Rössler and Litz75–Reference Friesecke, Lotze and Köhler77) report contradictory findings or do not have a satisfactory design. One important factor, highlighted by the study of Heller et al., is the dose of fish oil required to influence clinical outcomes. Overall the data available are suggestive of some clinical benefit from the inclusion of long-chain n-3 PUFA in parenteral nutrition regimens given to critically ill patients. However, only limited studies have been published and the inconsistency of findings limits translation to the clinic. Thus, further studies are required; clearly such studies need to be properly designed and adequately powered.
Guidelines with regard to the use of fish oil in parenteral nutrition
Very recently new guidelines for parenteral nutrition were issued by the European Society for Clinical Nutrition and Metabolism (ESPEN)(Reference Braga, Ljungqvist and Soeters6, Reference Singer, Berger and Van den Berghe7). The guidelines on parenteral nutrition in surgery state ‘… there is some evidence that inclusion of n-3 fatty acids in parenteral nutrition may benefit organ function and reduce length of stay in patients undergoing major surgery or admitted to the surgical ICU. However, these trends will need to be substantiated in adequately powered randomised trials’ and recommend ‘The optimal parenteral nutrition regimen for critically ill surgical patients should probably include supplemental n-3 fatty acids (Grade C)’(Reference Braga, Ljungqvist and Soeters6). The guidelines on parenteral nutrition in intensive care recommend ‘Addition of EPA and DHA to lipid emulsions has demonstrable effects on cell membranes and inflammatory processes (Grade B). Fish oil-enriched lipid emulsions probably decrease length of stay in critically ill patients (Grade B)’(Reference Singer, Berger and Van den Berghe7).
Use of fish oil in enteral nutrition in post-surgical and critically ill patients
Enteral formulas combining fish oil and arginine
Several enteral formulas using a combination of nutrients have been developed, typically including arginine, nucleotides and long-chain n-3 fatty acids. The majority of trials in surgical and critically ill patients have used IMPACT® (Nestle Nutrition, Gland, Switzerland) and a number of these studies reported immune and/or inflammatory outcomes (see Calder(Reference Calder78) for references). Most studies reporting circulating lymphocyte numbers and subsets, and circulating Ig concentrations showed little difference between IMPACT-treated patients and controls, although some studies reported benefits on phagocytosis, respiratory burst, lymphocyte proliferation, human leucocyte antigen-DR expression on monocytes and cytokine production(Reference Calder78). These effects could be due to any single specified nutrient (i.e. arginine, nucleotides, long-chain n-3 fatty acids) or to a combination of nutrients. Meta-analyses of controlled, randomised clinical studies using IMPACT or similar formulas have identified significant reductions in infections and length of hospital stay but these effects are more evident in surgical rather than critically ill patients(Reference Beale, Bryg and Bihari79–Reference Waitzberg, Saito and Plank83), and none of the meta-analyses shows a significant effect on mortality. Despite some clear statements to the contrary in the earlier meta-analyses(Reference Beale, Bryg and Bihari79–Reference Heyland, Novak and Drover81), concern has been raised that these formulas may actually be detrimental in the seriously ill(Reference Heyland, Dhaliwal and Drover84–Reference Suchner, Heyland and Peter86). This is because some studies of these formulas in critically ill patients reported increased mortality(Reference Beale, Bryg and Bihari79–Reference Heyland, Novak and Drover81). The source of the concern is the high arginine content, which is thought to drive excessive production of nitric oxide(Reference Suchner, Heyland and Peter86, Reference Zhou and Martindale87).
Enteral formulas combining fish oil, borage oil and antioxidants
Work in experimental animals indicated a benefit of the combination of fish oil and borage oil (the latter contains γ-linolenic acid) with regard to lung inflammation and damage in endoxaemia(Reference Mancuso, Whelan and DeMichele56, Reference Mancuso, Whelan and DeMichele57). OxEPA® (Abbott Nutrition, Columbus, OH, USA) is an enteral formula that contains a high level of fat (55% of energy), with the fat being a combination of 32% canola (i.e. low-erucic acid rapeseed oil), 20% fish oil, 20% borage oil, 25% MCT and 3% soyabean phospholipid. OxEPA also contains β-carotene, taurine and carnitine, and more vitamin C and vitamin E than standard enteral formulas. OxEPA was trialled for 7 d in patients with acute respiratory distress syndrome(Reference Gadek, DeMichele and Karlstad88, Reference Pacht, DeMichele and Nelson89). By 4 d of treatment the numbers of total leucocytes and of neutrophils in the alveolar fluid declined significantly in the n-3 fatty acid group and were lower than in controls(Reference Gadek, DeMichele and Karlstad88). Alveolar fluid IL-8 was lower in the n-3 fatty acid group compared with controls and LTB4 and TNF-α tended to be lower(Reference Pacht, DeMichele and Nelson89). Arterial oxygenation and gas exchange were also improved and the n-3 fatty acid-treated patients had a decreased requirement for supplemental oxygen, decreased time on ventilation support (11 v. 16·3 d; P=0·011) and a shorter length of stay in intensive care (12·8 v. 17·5 d; P=0·016)(Reference Gadek, DeMichele and Karlstad88). The total length of hospital stay tended to be shorter in the n-3 fatty acid group (29·4 v. 34·6 d; NS) and fewer patients developed new organ failure (4/51 v. 13/47; P=0·015)(Reference Gadek, DeMichele and Karlstad88). Mortality was 12% in the n-3 fatty acid group and 19% in the control group, but this difference was not statistically significant(Reference Gadek, DeMichele and Karlstad88). Since OxEPA not only provided fish and borage oils but also MCT, β-carotene, taurine and carnitine, and more vitamin C and vitamin E than the control formula, it is not possible to ascribe the benefits to any particular nutrient, although the anti-inflammatory effects seen are consistent with those of fish oil n-3 PUFA. Two more recent studies also report benefits from OxEPA in acutely ill patients(Reference Singer, Theilla and Fisher90, Reference Pontes-Arruda, Aragão and Albuquerque91). In one of these studies patients with acute lung injury received a control formula or OxEPA for 14 d(Reference Singer, Theilla and Fisher90). By days 4 and 7 patients receiving OxEPA showed improved oxygenation, a reduction in length of ventilation (160 v. 167 h; P<0·03) and a shorter ICU stay (12·8 v. 17·5 d; P=0·016), although there was no difference between the groups in mortality(Reference Singer, Theilla and Fisher90). In the second trial, OxEPA was used in ventilated patients with severe sepsis and septic shock(Reference Pontes-Arruda, Aragão and Albuquerque91). Patients receiving OxEPA had better oxygenation, more ventilator-free days (13·4 v. 5·8 d; P<0·001), fewer days in the ICU (4·6 v. 10·8 d; P<0·001), less development of new organ dysfunctions (38% v. 81%; P<0·001) and reduced 28-d mortality (33% v. 52%; P=0·037).
These three studies were recently combined in a meta-analysis(Reference Pontes-Arruda, Demichele, Seth and Singer5). This demonstrated a significant overall benefit of OxEPA v. control on requirement for ventilation (P<0·0001), ICU stay (P<0·0001), new organ failures (P<0·0001) and mortality (P=0·001).
Guidelines with regard to the use of fish oil in enteral nutrition
The ESPEN guidelines for use of enteral nutrition in surgical patients recommend ‘Use enteral nutrition with immuno-modulating substrates (arginine, n-3 fatty acids and nucleotides) perioperatively for patients undergoing major neck surgery for cancer, undergoing major abdominal cancer surgery or after severe trauma (Grade A)’(Reference Weimann, Braga and Harsanyi92). The guidelines for use of enteral nutrition in intensive care recommend ‘Immune modulating formulae (formulae enriched with arginine, nucleotides and n-3 fatty acids) are superior to standard enteral formulae in elective upper gastrointestinal surgical patients (Grade A), in patients with mild sepsis (Grade B), in patients with trauma (Grade A), in patients with acute respiratory distress syndrome (formulae containing n-3 fatty acids and antioxidants) (Grade B)’(Reference Kreymann, Berger and Deutz93). The latter recommendation was based upon a single study(Reference Gadek, DeMichele and Karlstad88), and as mentioned earlier two further supportive studies have now been published(Reference Singer, Theilla and Fisher90, Reference Pontes-Arruda, Aragão and Albuquerque91). The guidelines for enteral nutrition in intensive care also recommend ‘ICU patients with very severe illness who do not tolerate more than 700 ml enteral formula per day should not receive an immune-modulating formula enriched with arginine, nucleotides and n-3 fatty acids (Grade B)’(Reference Kreymann, Berger and Deutz93).
Summary and conclusions
Lipids traditionally used in parenteral nutrition are based on n-6 PUFA-rich vegetable oils like soyabean oil. This may not be optimal because it may present an excessive supply of linoleic acid. Alternatives to use of soyabean oil include its partial replacement by MCT, olive oil or fish oil, either alone or in combination. Lipid emulsions containing fish oil are well tolerated and without adverse effects in a wide range of adult patients. Fish oil-containing lipid emulsions have been used in parenteral nutrition in adult patients post-surgery (mainly gastrointestinal). This has been associated with alterations in patterns of inflammatory mediators and in immune function and, in some studies, a reduction in length of ICU and hospital stay. Perioperative administration of fish oil may be superior to post-operative. Parenteral fish oil has been used in critically ill adults. Here the influence on inflammatory processes, immune function and clinical endpoints is not clear, since there are too few studies and those that are available report contradictory findings. Fish oil is included in combination with other nutrients in various enteral formulas. In post-surgical patients and in those with mild sepsis or trauma, there is clinical benefit from a formula including fish oil and arginine. A formula including fish oil, borage oil and antioxidants has demonstrated marked benefits on gas exchange, ventilation requirement, new organ failures, ICU stay and mortality in patients with acute respiratory distress syndrome, acute lung injury or severe sepsis.
Acknowledgements
The author has received speaking fees from B. Braun, Fresenius Kabi, Baxter Healthcare, Abbott Nutrition and Nestle, participated in the Baxter Healthcare Global Advisory Board in 2008, and has received research funding from B. Braun.



