Within this supplement devoted to innovation, it is, perhaps, unusual to be considering the description of revelations in cardiac morphology. Those investigating the structure of the normal and congenitally malformed heart in the 21st century, in terms of gross morphology, can do little more than those such as LeonardoReference Wells and Crowe1. He, and fellow morphologists, initially revealed most of the secrets of normal cardiac structure by careful dissection of hearts obtained at cardiac autopsy. In the light of centuries of subsequent investigation, it is surprising that so many issues regarding the structure of not only congenitally malformed hearts, but also the normal heart, remain contentious. To take but one example, interpretations vary with regard to the precise nature and description of that part of the subpulmonary infundibulum adjacent to the aortic root. Much of this discussion devolves on the topology of the conal, or muscular outlet, septum, a feature that underscores some categorizations of holes between the ventricles.Reference Van Praagh, Geva and Kreutzer2 The very fact that the pulmonary valve can be removed in its entirety, along with a free-standing infundibular sleeve, for subsequent use as an autograft in the Ross procedure, however, suggests that any muscular component separating the cavities of the subaortic and subpulmonary outflow tracts must be of limited dimensions in the normally structured heart.Reference Merrick, Yacoub, Ho and Anderson3, Reference Anderson and Brown4 Perhaps the most important innovatory role of the morphologist, therefore, is to seek to discover the reasons for ongoing differences in interpretation, and hopefully to resolve them, combining in this endeavour with those expert in visualizing cardiac structures during life. It is also to be hoped that morphologists will accept the necessity to change any previous concepts revealed, with the passage of time, to be less than perfect. In this review, therefore, we will explore the innovative potential of describing the topology and morphology of the normal ventricular septum, showing how such analysis potentially provides the basis for a non-contentious categorization of the various forms of holes that can exist between the cavities of the ventricles.
Categorisation of Holes between the Ventricles
Several popular, but disparate, approaches exist for the categorisation of ventricular septal defects.Reference Jacobs, Burke, Quintessenza and Mavroudis5 The time-honoured approach is to place such lesions in one of 4 groups.Reference Wells and Lindesmith6 Those making up the first category are the ones occupying the area, in the normal heart, usually closed by the ventricular component of the membranous part of the septum. Hence the traditional title of membranous defects. The second group is made up of the various holes which, when viewed from the right ventricular aspect, have exclusively muscular borders. These holes are well recognised as being found in various parts of the muscular ventricular septum. The third group comprises the holes that are found immediately adjacent to the leaflets of the pulmonary valve. These holes, in the past, when compared to the membranous defects, have often been described as being supracristal, the membranous ones considered to be infracristal. The final group is made up of so-called atrioventricular canal defects, albeit that the holes falling within this group are the least well-defined.Reference Wells and Lindesmith6
The second popular categorisationReference Van Praagh, Geva and Kreutzer2 is a modification of the time-honoured approach, the supracristal defects being interpreted to represent conal hypoplasia. In addition, defects characterised by malalignment between the conal septum and the remainder of the muscular ventricular septum were grouped together as conoventricular defects,Reference Van Praagh, Geva and Kreutzer2 albeit that a further modification based primarily on the location of the holes expanded the definition of these conoventricular entities.7
The third popular categorisation emerged from Europe.Reference Soto, Becker, Moulaert, Lie and Anderson8 A key point of this approach was to group defects together on the basis of the anatomic nature of their borders as seen from the right ventricle, acknowledging also the importance of their different locations relative to the components of the right ventricle, as well as describing septal malalignment, when that feature was present. On the basis of the anatomic nature of these borders as viewed from the right ventricle, the European investigators pointed out that all ventricular septal defects could be placed in one of 3 groups, irrespective of how they opened into the different parts of the right ventricle. The defining feature for the first group was that part of the border of the hole was formed by fibrous continuity between the leaflets of an arterial valve and an atrioventricular valve, or in the setting of double outlet ventricle, between the leaflets of the 2 atrioventricular valves. Since the membranous septum formed part of the perimeter of these defects, the European investigators chose to describe these defects as being perimembranous. The second group was made up of the holes with exclusively muscular borders. The defining feature of the third group was the presence of fibrous continuity between the leaflets of the arterial valves. Included in this group were also those with the superior border, when seen from the right ventricle, made up by the leaflets of a common truncal valve.Reference Soto, Becker, Moulaert, Lie and Anderson8 To determine whether the differences between these approaches to categorisation reflect different anatomic interpretations as opposed to semantics, it is helpful to review the structure of the normal ventricular septum.
The Building Blocks of the Normal Ventricular Septum
All of those providing categorisations of congenitally malformed hearts have recognised that knowledge of normal structure is a pre-requisite for accurate differentiation. Part of the innovatory role of the morphologist, thereore, is to ensure that such concepts keep pace with newly acquired information. It has long been known that the normal septum possesses muscular and fibrous components, the latter part being the membranous septum. Discussions continue regarding the extent of the muscular septum, and how its topology is best described. Morphologists can best help their clinical colleagues in this area by preparing their autopsied specimens so as to replicate the images now obtained routinely during life. An image taken to replicate the subcostal oblique cut obtained by the echocardiographer through the right atrioventricular junction, if taken so that it extends into the aortic root, shows that very little of the normal muscular ventricular septum interposes between the ventricular inlets (Fig. 1), even though often considered to be an inlet septum. This is because, in hearts with separate right and left atrioventricular junctions, the subaortic outflow tract interposes between the orifice of the mitral valve and the ventricular septum, a feature well demonstrated in both four-chamber (Fig. 2) and short axis sections taken to replicate echocardiographic views. Close-ups of this area also show how the septal leaflet of the tricuspid valve crosses the membranous septum, dividing it into atrioventricular and interventricular components (Fig. 3). Dissections made to replicate the anatomy demonstrated by the clinician when producing parasternal long axis echocardiographic sections through the left ventricle show how the leaflets of the pulmonary valve are lifted away from the base of the ventricular mass by the free-standing subpulmonary infundibular sleeve (Fig. 4). The presence of this sleeve can be confirmed by replicating the dissection made by the surgeon during the Ross procedure, thus revealing the collar of infundibular musculature that remains when the pulmonary valvar leaflets themselves have been removed from the heart (Fig. 5). This free-standing muscular infundibular sleeve was not initially recognised by the European group when introducing their suggested classification for holes between the ventricles.Reference Soto, Becker, Moulaert, Lie and Anderson8 In their initial description, therefore, they wrongly suggestedReference Soto, Becker, Moulaert, Lie and Anderson8 that defects opening towards the outlet of the right ventricle excavated the outlet septum. In reality, such defects rather serve to reveal the existence of the muscular outlet septum, which can then be recognised as the muscular structure interposed between the ventricular outflow tracts.

Figure 1 This normal heart has been sectioned to replicate the subcostal oblique echocardiographic cut through the right ventricular inlet. It shows how the inferior part of the muscular septum, by virtue of the wedged position of the subaortic outflow tract, separates the inlet of the right from the outlet of the left ventricle (double headed arrow).
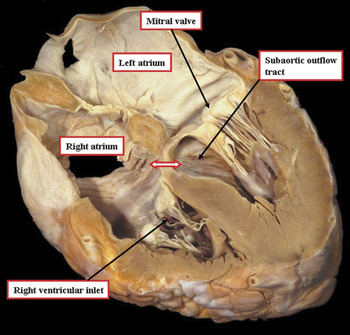
Figure 2 This cut through a normal heart is made to replicate the echocardiographic four chamber view incorporating the left ventricular outflow tract. It shows how the wedged location of the aortic valve lifts the leaflets of the mitral valve away from the muscular ventricular septum so that the inlet of the right ventricle is separated from the left ventricular subaortic outlet. Note the location of the atrioventricular component of the membranous septum (double headed arrow).

Figure 3 The image shows the postero-inferior part of the subaortic outflow tract of the normal heart. The septal leaflet of the tricuspid valve is attached across the right side of the membranous septum, dividing it into atrioventricular (red double headed arrow) and interventricular (green double headed arrow) components.

Figure 4 This normal heart has been dissected to replicate the parasternal long axis section obtained echocardiographically, but in addition the space between the free-standing subpulmonary infundibulum and the aortic root has been cleaned to show the extent of the muscular infundibular sleeve (white bracket).

Figure 5 The base of this normal heart has been dissected by removing the pulmonary valve, showing the nature of the free-standing infundibular sleeve that lifts the valvar leaflets away from the ventricular base, making possible the Ross procedure. Note the location of the origin of the first septal perforating artery (star) from the anterior interventricular branch of the left coronary artery.
This information also focusses attention on the components of the “crista supraventricularis”, which can be translated as the supraventricular crest. This muscular crest interposed between the leaflets of the tricuspid and pulmonary valves in the roof of the normally structured right ventricle, inserts between the limbs of a prominent septal trabeculation, the latter known either as the septal band or the septomarginal trabeculation (Fig. 6a). The septal band, which reinforces the surface of the muscular ventricular septum, is positioned within the cavity of the right ventricle rather than in its roof. If described literally, therefore, it should not be considered as part of the supraventricular crest. Indeed, in many hearts a line of union can be recognised at the point where the supraventricular crest itself inserts between the limbs of the septal band. Dissection of normal hearts then shows that the larger part of the supraventricular component is composed of the free wall of the right ventricle, described also as the ventriculo-infundibular fold, which supports distally the free-standing muscular infundibular sleeve. By making careful dissections between the limbs of the septal band, it is possible to create a small channel that leads to the outflow tract of the left ventricle (Fig. 6b). This small part is the muscular outlet septum. In the normal heart, nonetheless, there are no landmarks to show where this potentially septal component stops, and where the ventriculo-infundibular fold begins.

Figure 6 The photographs show (a) the roof of the normal right ventricle, in a different heart from the one shown in Figure 6, with the supraventricular crest inserting between the limbs of the septomarginal trabeculation (SMT), or septal band. The dotted line shows the line of insertion. Note also the septoparietal trabeculations (SPT). The dissection (b) shows how a small part of the muscle between the limbs of the septomarginal trabeculation can be removed to create a channel leading to the left ventricle, representing the muscular outlet septum, but there are no anatomical landmarks in the normal heart to distinguish the union of this septal component with the larger part of the crest, which is formed by the parietal wall of the right ventricle in the inner heart curvature.
Perhaps paradoxically, it is easier to recognise these components in their own right in hearts with holes between the ventricles. The septal component of the supraventricular crest, for example, can then be recognised in some of these hearts as the outlet, conal, or infundibular septum, albeit still supporting a free-standing infundibular sleeve when traced distally. The ventriculo-infundibular fold can also be recognised in many of these hearts, since cuts across the inner curvature show that any muscle interposed between the leaflets of an arterial valve and an atrioventricular valve must be part of this structure (Fig. 7).Reference Anderson, Becker and Van Mierop9 Attention to these features then shows the difference between the so-called supracristal and infracristal defects.
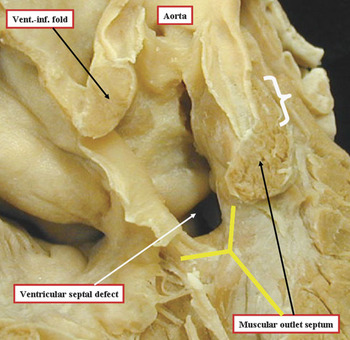
Figure 7 The photograph is of a heart with tetralogy of Fallot, albeit an unusual specimen in that the overriding aorta is connected predominantly to the right ventricle, and there is a completely muscular subaortic infundibulum. It has been dissected to show the nature of the muscular structures surrounding the ventricular septal defect, which opens to the right ventricle between the limbs of the septal band, or septomarginal trabeculation (yellow Y). Note that the musculature of the inner heart curvature, or the ventriculo-infundibular (vent.-inf.) fold interposes between the leaflets of the aortic and tricuspid valves. The muscular outlet septum separates the pathways from the cavity of the right ventricle extending towards the aortic and pulmonary valves, albeit that the pulmonary valvar leaflets, as in the normal heart, are supported by a free-standing muscular infundibular sleeve (white bracket).
In the holes considered to be infracristal, the defect opens into the right ventricle between the limbs of the septal band, and is roofed by the muscular outlet or conal septum, now recognisable in its own right as the muscular structure interposed between the outflow tracts. This muscular structure, however, along with the free-standing subpulmonary infundibulum, is lacking in the hearts said to have supracristal defects. In these latter defects, the structure relative to which the defect can be considered supracristal is the septal band. The alleged supracristal and infracristal defects, however, are related in comparable fashion to the limbs of the septal band (Compare Fig 8a, b). Thus, there is no logical foundation for distinction of these holes between the ventricles as being supracristal as opposed to infracristal. By taking an innovative approach to the demonstration of cardiac morphology, therefore, and by noting those features which have become obvious over the recent decades with the advent of tomographic techniques for imaging, seeking at the same time to demonstrate the morphology as seen by the clinician working with the living patient, it is possible to resolve at least one of the controversies relating to the categorisation of holes between the ventricles. Can such approaches also unify the other popular systems for categorisation?

Figure 8 The pictures show how it is inappropriate to seek to distinguish the illustrated defects as being infracristal (a) and supracristal (b). In panel a, the defect, which is variously considered to be perimembranous or conoventricular, is cradled between the limbs of the septal band (yellow Y), but roofed by the muscular outlet septum. Note that the parietal extension of the outlet septum is continuous with the muscular inner heart curvature, also known as the ventriculo-infundibular fold. It is the relationship to the central part of the muscular outlet septum, interposed between the pathways leading to the aortic and pulmonary valves. that justifies the description of the defect as being “infracristal”. Panel b shows the defect characterised by fibrous continuity between the leaflets of the aortic and pulmonary valves, also known as the doubly committed, or conal hypoplasia, defect. In hearts of this type, there is absence of the muscular outlet septum, along with the free-standing subpulmonary infundibulum. The defect, however, continues to open to the right ventricle between the limbs of the septal band. The extensive muscular postero-inferior rim to the defect exists because the posterior limb of the septal band has fused with the ventriculo-infundibular fold. In this situation, it is this muscular bundle, notably the posterior limb of the septal band, that is deemed to represent the “crista”. If using this definition, nonetheless, the hole shown in panel a is also supracristal!!

Figure 9 The defect opens to the inlet of the right ventricle, being cradled within the limbs of the septomarginal trabeculation, or septal band (yellow Y). Although not readily visible from the right ventricle, part of the border of the hole is made up of fibrous continuity between the leaflets of the aortic, tricuspid, and mitral valves. This feature, along with the separate nature of the mitral valve, which guards a discrete left atrioventricular junction, is best seen from the left side (see Fig. 10a). The picture was taken by Professor Jeong Seo, of the University of Seoul, South Korea, and is reproduced with his permission.
Seeking Unity in the Description of Holes between the Ventricles
When comparison is made of the features used in the different categorisations to distinguish the various holes, it emerges that, when seeking to distinguish the different types of defect, each approach concentrates on a different morphological feature. Apart from the approach taken by the European school,Reference Soto, Becker, Moulaert, Lie and Anderson8 the other systemsReference Van Praagh, Geva and Kreutzer2, Reference Wells and Lindesmith6, 7 use as their primary defining feature the positions taken by the different holes relative to the components of the normal ventricular septum. The system introducing the importance of the conusReference Van Praagh, Geva and Kreutzer2 places additional emphasis on malalignment and hypoplasia of the muscular septum interposed between the subaortic and subpulmonary outlets. The European system,Reference Soto, Becker, Moulaert, Lie and Anderson8 in contrast, while not ignoring the location of the holes relative to the right ventricle, places greater emphasis on the nature of the border of the hole as seen from the right ventricle. When using this feature, the Europeans argued that holes should be grouped together even if they opened to different parts of the right ventricle. The reason for this was that knowledge of the anatomic borders of the defect provided crucial information concerning the location of the atrioventricular bundle, or atrioventricular conduction axis, as seen during surgical correction.Reference Soto, Becker, Moulaert, Lie and Anderson8 If this information concerning the anatomic nature of the borders is combined with the location of the holes relative to the normal septum, it is possible to distinguish between the various holes that open to the same part of the right ventricle, yet are phenotypically discrete.
Holes opening primarily to the inlet of the right ventricle
At least 4 phenotypically discrete holes open towards the right ventricular inlet. A fifth type has been described,Reference Soto, Ceballos and Kirklin10 but to date none of us has seen this fifth variant in clinical practise. The commonest type of defect that opens to the right ventricular inlet (Fig. 9) is the one that possesses, as part of its direct borders, fibrous continuity between the leaflets of the aortic, mitral, and tricuspid valves (Fig. 10a). Incorporated in this fibrous area is the atrioventricular component of the membranous septum. In many defects of this type, the interventricular component of the membranous septum is often seen as a triangular fibrous flap reinforcing the postero-inferior margin as seen from the left ventricle. When the Europeans produced their classification,Reference Soto, Becker, Moulaert, Lie and Anderson8 they described these defects as being perimembranous. This word was chosen for description because, when seen from the left ventricle, the hole was seen to extend around the components of the membranous septum, which formed part of its perimeter. The posterior and inferior borders of the hole, when seen from the left ventricle, therefore, were made up of fibrous tissue, and incorporated the atrioventricular component of the membranous septum, even when holes of this type opened to the outlet rather than the inlet of the right ventricle (Fig. 10b).

Figure 10 When the hole shown in Figure 9 is seen from the left side (Fig. 10a), then it can be recognised, first, that there is a separate left atrioventricular junction, guarded by the mitral valve, so the heart does not have an atrioventricular canal in the sense of a common atrioventricular junction. It can also be seen that the postero-inferior border of the defect is made up of fibrous continuity between the leaflets of the tricuspid, aortic, and mitral valves. This feature is also seen when defects of this kind open to the right ventricular outlet, as shown in Figure 10b. In this specimen, the heart has been transilluminated to show that the area of fibrous continuity incorporates the atrioventricular part of the membranous septum. The defect itself, therefore, in that it extends around the remnant of the membranous septum, is perimembranous in both the illustrated hearts. The images were taken by Professor Jeong Seo, from the University of Seoul, South Korea, and are reproduced with his permission.
The holes of this kind opening to the inlet of the right ventricle had previously been considered to be comparable to the septal deficiencies seen in atrioventricular canal malformations.Reference Van Praagh, Geva and Kreutzer2, Reference Wells and Lindesmith6, 7 One of the phenotypic features of the atrioventricular canal defect, however, is a common atrioventricular junction.Reference Anderson, Ho, Falcao, Daliento and Rigby11 The commonest type of hole opening between the ventricular inlets, in contrast, has separate right and left atrioventricular junctions, with the aortic outflow tract wedged between them (Fig. 10a). Such holes, therefore, cannot be atrioventricular canal malformations in the sense that they possess a common atrioventricular junction. It could be argued that the hole itself is directly comparable to the ventricular component of an atrioventricular septal defect. This premise also fails to stand rigorous scrutiny. In hearts with an atrioventricular septal defect and common atrioventricular junction, or atrioventricular canal malformations, there is marked disproportion between the inlet and outlet dimensions of the ventricular septum (Fig. 11a), the aortic outflow tract is unwedged relative to the common atrioventricular junction, and the left atrioventricular valve is a trifoliate structure. In the hearts with defects bordered by fibrous continuity between the aortic and left atrioventricular valves, but with separate atrioventricular junctions, the inlet and outlet dimensions of the septum are the same (Fig. 11b), the aorta is normally wedged between the atrioventricular junctions, and the left valve is a bifoliate structure. The importance of recognising this phenotype is that, when the postero-inferior border is fibrous, the atrioventricular bundle, arising from an atrioventricular node located at the apex of the triangle of Koch, runs postero-inferiorly relative to the hole, and is seen to the right hand of the surgeon operating through the tricuspid valve (Fig. 12).
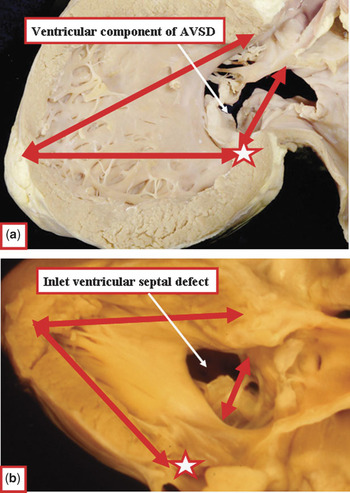
Figure 11 The images compare the phenotypic features of the ventricular component of the defect seen in atrioventricular canal malformations (Fig. 11a) and the hole bordered by fibrous continuity between the leaflets of the aortic and tricuspid valves in hearts with separate atrioventricular junctions (Fig. 11b). In addition to the differences in junctional morphology, the holes differ with regard to the inlet-outlet dimensions of the ventricular septum (long arrows), the extent of the hole relative to the crux (short arrows and star), and the degree of unwedging of the aortic valve.

Figure 12 When defects having fibrous continuity between the leaflets of the aortic and tricuspid valves as part of their border open to the inlet of the right ventricle, so-called perimembranous defects, the conduction axis is always related to their postero-inferior margin, to the right hand of the surgeon operating through the tricuspid valve. In contrast, the conduction axis runs antero-superior, or to the left hand of the surgeon, when a defect opens to the inlet of the right ventricle but with exclusively muscular borders.
The second important defect opening to the inlet of the right ventricle is distinguished because it has exclusively muscular borders. Such holes are well described as being muscular inlet defects. They are recognised by the echocardiographer because the persisting musculature of the septum preserves the normal off-setting of the leaflets of the mitral and tricuspid valves (Fig. 13). The importance of recognising this hole is that the conduction axis runs antero-cephalad, and is to the left hand of the surgeon operating through the tricuspid valve (Fig. 12).
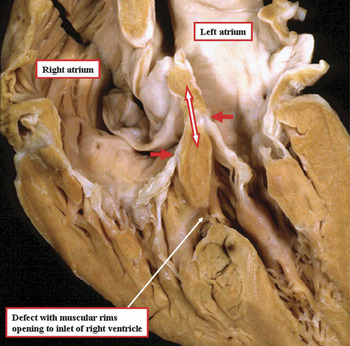
Figure 13 The heart has been sectioned to replicate the echocardiographic four chamber cut, and shows a defect with exclusively muscular rims opening to the inlet of the right ventricle. Note the retention of the off-setting of the hinges of the leaflets of the atrioventricular valves. The double headed arrow shows the course taken by the atrioventricular conduction axis from the apex of the triangle of Koch to the strip of ventricular musculature separating the defect from the plane of atrioventricular insulation, such that the atrioventricular bundle passes antero-superiorly relative to the inlet defect itself (see also Fig. 12).
There is then a third type of defect opening to the inlet of the right ventricle. This is the hole associated with straddling and overriding of the tricuspid valve. In this type of hole, the muscular septum extends across the full width of the right atrioventricular junction (Fig. 14a). In the past, this type of defect has also been considered to be atrioventricular canal in type.Reference LaCorte, Fellows and Williams12, Reference Pessotto, Padalino, Rubino, Kadoba, Büchler and Van Praagh13 Like the hole with fibrous postero-inferior borders opening to the right ventricular inlet, however, it cannot be an atrioventricular canal defect, since apart from having separate right and left atrioventricular junctions (Fig. 14b), the hearts containing such defects have none of the other phenotypic features of atrioventricular canal malformations (Fig. 15). An additional reason for segregating this defect as a distinct phenotype is that the atrioventricular bundle arises anomalously from a postero-inferior node, which does not arise at its anticipated site within the apex of the triangle of Koch (Fig. 16). Failure to recognise this feature courts the danger of producing iatrogenic damage to the conduction axis during surgical repair.

Figure 14 The images, from different hearts, show the essence of the inlet defect associated with straddling tricuspid valve. Panel a shows how, in the setting of overriding of the tricuspid valvar orifice, the atrioventricular junction extends along the full length of the muscular ventricular septum. Panel b shows the malalignment of the atrial and muscular ventricular septums (stars), albeit in the setting of separate right and left atrioventricular junctions (double headed arrows).

Figure 15 The image shows the left ventricular aspect of a heart having straddling and overriding of the tricuspid valve. The heart has none of the phenotypic features of atrioventricular canal malformations. The inlet and outlet dimensions of the ventricular septum are the same (red arrows), the aorta is wedged between the left and right atrioventricular junctions, and the left atrioventricular valve is a bifoliate structure. Note that the postero-inferior aspect of the muscular septum, obscured by the straddling leaflets of the tricuspid valve, does not extend to the crux (star).

Figure 16 The cartoon shows the grossly abnormal situation of the atrioventricular conduction axis when there is straddling and overriding of the tricuspid valve.
Yet a fourth type of hole between the ventricles then opens to the inlet of the right ventricle. This is the true atrioventricular canal defect, but with shunting across the septal defect confined at ventricular level. The hearts containing these defects, therefore, have all the phenotypic features of the atrioventricular canal malformations. Shunting is confined at ventricular level because the bridging leaflets of the common atrioventricular valve are firmly attached to the superior margin of the atrioventricular septal defect. The scooped-out ventricular septum inserts at the crux (Fig. 17), with the atrioventricular conduction axis arising from a node as expected for other types of atrioventricular septal defect with common atrioventricular junction.
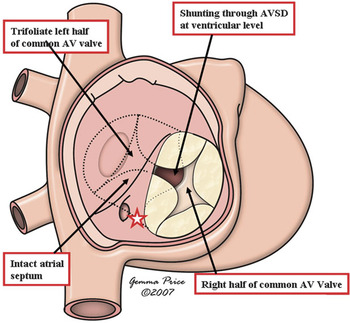
Figure 17 The cartoon shows the essential features of an atrioventricular septal defect with common atrioventricular junction, but with shunting confined at ventricular level because of the attachment of the bridging leaflets to the underside of the atrial septum. The star shows the site of the atrioventricular node at the crux of the heart.
It has been suggested that a fifth type of defect could open to the inlet of the right ventricle. This is the hole allegedly roofed by the conjoined leaflets of the mitral and tricuspid valves, with separate atrioventricular junctions, but separated antero-superiorly by muscular tissue from an intact membranous septum.Reference Jacobs, Jacobs, Mavroudis, Lacour-Gayet and Tchervenkov14 We have never seen such a heart, either at autopsy or during clinical examination. We believed we might recently have seen an example following the echocardiographic interrogation (Fig. 18), but during surgical repair, a small muscle bar was found along the superior margin of the hole, showing that in reality it was a muscular inlet defect.

Figure 18 The echocardiographic images show a defect initially interpreted as being roofed by fibrous continuity between the leaflets of the mitral and tricuspid valves (left hand panel), yet separated by muscle from the area of the membranous septum (right hand panel). At surgical repair, however, a small rim of muscle (double headed red arrow) was found separating the superior margin of the defect from the hinge of the tricuspid valve (yellow arrow), indicating that the defect was a muscular hole opening to the inlet of the right ventricle. The green arrow shows the hinge of the mitral valve.
Holes opening to the right ventricle at the site of the membranous septum
When considering the overall population of patients with holes between the ventricles, it is likely that most have muscular defects. For those requiring closure, however, the commonest variant is the hole that opens into the right ventricle at the site usually filled by the interventricular component of the membranous septum, albeit that the hole occupies an area appreciably larger than the space usually filled by the interventricular component of the membranous septum (Compare Figs 2 and 19). This type of hole is by far the commonest congenital defect coded by those making surgical returns to the databases maintained by the Society of Thoracic Surgeons and the European Association of Cardiothoracic Surgeons.Reference Jacobs, Jacobs, Mavroudis, Lacour-Gayet and Tchervenkov14 In the time-honoured classification of defects, this type of hole was described as being membranous, the presumption being that it reflected absence of the interventricular part of the membranous septum. As was already pointed out by Becu and associates,Reference Becu, Fontana, DuShane, Kirklin, Burchell and Edwards15 and as discussed above, the hole itself is appreciably larger than the fibrous septal component itself. Furthermore, as shown in Figure 19, this part of the septum is oftentimes present as a triangular fibrous flap reinforcing the postero-inferior margin of the defect. The atrioventricular component of the membranous septum, furthermore, forms part of the fibrous continuity between the leaflets of the aortic and tricuspid valves, this being the phenotypic feature of the defect. The defect exists because of deficiency of the ventricular septal musculature in the environs of the hole usually closed by the membranous septum, rather than failure to form the fibrous septal component. For this reason, as has been discussed above, the European groupReference Soto, Becker, Moulaert, Lie and Anderson8 proposed that the hole be described as a perimembranous defect. For better or worse, and despite arguments concerning the appropriateness of this term, the term is now firmly entrenched in the paediatric cardiac lexicon. Recognition of the fibrous continuity existing between the leaflets of an arterial and atrioventricular valve as part of the margin of these defects conveys the information that the atrioventricular bundle will be related to their postero-inferior corner, and will be to the right hand of the surgeon operating through the orifice of the tricuspid valve. This holds good whether the defect opens directly beneath the inner heart curvature, as shown in Figure 19a, or extends so as to open more to the inlet (Figs 9, 10a,), or to the outlet (Fig 10b) of the right ventricle. The only exception to this rule in hearts with concordant atrioventricular connections occurs when there is atrioventricular septal malalignment, this being the phenotypic feature of the inlet defect described above which has, as its phenotypic feature, straddling and overriding of the tricuspid valve (Fig. 14).

Figure 19 Panel a shows the commonest type of hole between the ventricles requiring therapeutic closure. It opens into the right ventricle at the site usually occupied by the interventricular component of the membranous septum, being floored by the limbs of the septomarginal trabeculation, or septal band (yellow Y). In this heart, the interventricular component of the membranous septum is well formed, and hangs down as the triangular membranous flap. The phenotypic feature of the defect is fibrous continuity between the leaflets of the aortic and tricuspid valves in the postero-inferior margin of the defect when viewed from the right ventricle. The section replicating the echocardiographic four chamber cut (Panel b) shows that the atrioventricular component of the membranous septum (red dotted oval) is part of this fibrous continuity (see also Fig. 10b).
Defects opening to the outlet of the right ventricle
As with the defects opening primarily to the inlet of the right ventricle, defects with markedly different phenotypes can open in direct relationship to the ventricular outflow tracts. The commonest of these is the hole that possesses, as part of its border, fibrous continuity between the leaflets of the aortic and tricuspid valves. Such holes can open directly beneath the subpulmonary infundibulum even in the absence of marked overriding of the right ventricular cavity by the leaflets of the aortic valve. More usually, such defects with a partly fibrous margin, described as being perimembranous in the European categorisation,Reference Soto, Becker, Moulaert, Lie and Anderson8 have associated malalignment between the muscular outlet septum and remainder of the ventricular septum. The defects open to the right ventricle between the limbs of the septal band, but in the absence of subpulmonary infundibular obstruction (Fig. 20). It was defects of this type that were emphasised by EisenmengerReference Eisenmenger16 as frequently setting the scene for the development of pulmonary hypertension. The feature of malalignment between the outlet septum and the remainder of the muscular septum, of course, is the major phenotypic feature of the conoventricular defect, seen also in association with infundibular muscular stenosis in the setting of tetralogy of Fallot.Reference Anderson and Weinberg17 As is also the case in tetralogy of Fallot, such conoventricular defects can possess a different phenotype.

Figure 20 The heart shown in Figure 8a has been sectioned to replicate the subcostal oblique echocardiographic cut through the right ventricular outlet. It shows the phenotypic feature of the defect, namely fibrous continuity between the leaflets of the aortic and tricuspid valves. In this heart, however, unlike the one shown in Figure 19, there is additional malalignment of the muscular outlet septum, albeit in the absence of infundibular obstruction (white bracket). Note that the defect opens between the limbs of the septal band (yellow Y). Note also the persisting muscular infundibular sleeve supporting the leaflets of the pulmonary valve (red dotted line).
This second phenotypic type of conoventricular defect, opening beneath the ventricular outlets, has exclusively muscular borders when viewed from its right ventricular aspect (Fig. 21). This is because of the fusion of the posterior limb of the septal band with the ventriculo-infundibular fold, producing a muscular bar which interposes between the hinges of the leaflets of the aortic and tricuspid valves. The aortic valvar leaflets themselves are partially supported within the right ventricle, as is also the case in the conoventricular defect with fibrous continuity between the leaflets of the aortic and tricuspid valves. The defect itself continues to open to the right ventricle between the limbs of the septomarginal trabeculation (Fig. 21). Defects can also open to the right ventricular outlet with exclusively muscular rims in the absence of malalignment between the muscular outlet septum and the remainder of the muscular ventricular septum, reflecting some degree of conal hypoplasia (Fig. 22).

Figure 21 The muscular outlet septum is hypoplastic in this heart, but is also malaligned relative to the remainder of the muscular septum, which is reinforced by the limbs of the septal band (yellow Y). This conoventricular defect, therefore, is also a muscular outlet defect, with the fusion of the ventriculo-infundibular fold and the posterior limb of the septal band producing a muscular bar (star) that protects the atrioventricular conduction axis.

Figure 22 This muscular defect opens to the outlet of the right ventricle. The aortic valvar leaflets are seen through the defect, but there is no malalignment of the hypoplastic muscular outlet septum. The posterior limb of the septomarginal trabeculation fuses with the inner heart curvature to produce a muscular bar that protects the atrioventricular conduction axis.
The final type of defect opening to the outlet of the right ventricle exhibits an entirely different phenotype. This is the hole that is roofed by fibrous continuity between the leaflets of the aortic and pulmonary valves. It exists because of absence of not only the muscular outlet septum, but also the component of the subpulmonary infundibulum that normally interposes between the infundibular cavity and the aortic root. The outlet septal component itself can sometimes be visualised as a fibrous raphe beneath the conjoined leaflets of the arterial valves. Holes of this type are also found when there is a common arterial trunk exiting from the ventricular mass, but then there is a common arterial valve, rather than separate aortic and pulmonary valves, guarding the common ventriculo-arterial junction. In most defects of this type, which are doubly committed and juxtaarterial, a muscular rim is formed postero-inferiorly by fusion of the posterior limb of the septal band with the ventriculo-infundibular fold (Fig. 23a). A minority of these holes, nonetheless, can extend so as to be bordered by fibrous continuity between the leaflets of the arterial and atrioventricular valves, and hence also be perimembranous. In the latter setting, the atrioventricular bundle is exposed in the fibrous postero-inferior margin of the defect (Fig. 23b).

Figure 23 The 2 holes shown in panels a and b both exist because of failure of formation of the free-standing muscular subpulmonary infundibulum, along with the muscular outlet septum. Hence, the defects are doubly committed and juxtaarterial, being roofed by the conjoined leaflets of the arterial valves. In the lesion shown in Panel a, however, the ventriculo-infundibular fold fuses with the posterior limb of the septal band (yellow Y), producing a muscular bar that protects the atrioventricular conduction axis. In the heart shown in Panel b, in contrast, the defect extends so that there is fibrous continuity between the leaflets of not only the arterial valves, but also the aortic and tricuspid valves.
Ventricular septal defects versus interventricular communications
As we have discussed, several features have been used to categorise the different types of holes between the ventricles. The differences currently existing between the systems proposed for categorisation reflect the feature, or features, chosen as the primary distinguishing criterion. Full description, and consensus in definition, can be achieved simply by taking note of all these features. In all the categorisations emphasised in our review, the hole is defined on the basis of its margins as seen from the morphologically right ventricle. This approach in itself can potentially produce problems, the more so if the hole described as the ventricular septal defect is considered to represent the same plane in space as the interventricular communication.Reference Anderson, Becker and Tynan18 This is because, when there is overriding of an arterial valve, the interventricular communication, if defined precisely, is the direct continuation of the plane of the long axis of the ventricular septum. The cranial margin of this plane cuts through the leaflets of the overriding arterial valve. This plane of space, of course, is not that which is closed so as to restore septal integrity. It is the right ventricular margin of the cone of space subtended by the leaflets of the overriding valve to the crest of the muscular septum that is closed so as to restore septal integrity. It is this plane of space closed to restore septal integrity, therefore, that is described as the ventricular septal defect. The term “ventricular septal defect”, however, is used predominantly by those speaking anglo-saxon languages. We presume that the same hole, rather than the direct continuation of the long axis of the muscular ventricular septum, would be described as the interventricular communication by those using vernacular romantic languages, such as French, Italian, Spanish, and Portuguese (Fig. 24).
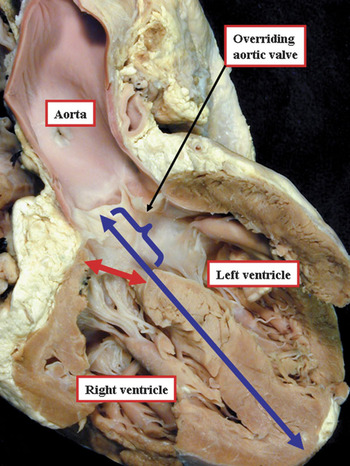
Figure 24 The section is a simulated four chamber cut through a heart with an overriding aortic valve. The plane separating the ventricular cavities is the direct continuation of the long axis of the muscular ventricular septum (blue line). The upper margin of this line (bracket) is, therefore, the plane of space representing the true interventricular defect. It is the plane shown by the red double headed arrow, however, that we choose to describe as the ventricular septal defect, which is usually considered synonymous with the interventricular communication as described in the French, Spanish, and Italian languages.
This apparent semantic problem, unlikely to create problems when accounting for so-called “isolated” defects, can produce practical difficulties when describing the arrangement in the setting of double outlet right ventricle. When both arterial trunks arise from the right ventricle, the hole between the ventricles functions as the outlet for the morphologically left ventricle. This hole is also the interventricular defect. It is never closed during therapeutic repairs aimed at restoring biventricular communications. Instead, this interventricular communication is tunneled to one or other of the subarterial outlets. It would be a disaster were the hole to be closed surgically in this setting, unless the surgeon also created a new outlet from the left ventricle. In most instances, a patch is usually placed from the crest of the muscular ventricular septum to the underside of the muscular outlet septum, thus effectively restoring septal integrity (Fig. 25).

Figure 25 The cartoon shows the potential problem in describing the hole between the ventricles in the setting of double outlet right ventricle. The interventricular communication, shown by the double headed red arrow, is the outlet for the left ventricle (LV). This hole is not closed, however during therapeutic repair. Instead, the hole is tunneled to one or other of the subarterial outlets, depending on the relationships of the aorta and the pulmonary trunk. The “ventricular septal defect”, therefore, is never closed during surgical repair of double outlet right ventricle, unless this defect is defined as the plane between the crest of the ventricular septum and the underside of the right ventricular outlet septum (blue oval).
Conclusions
In terms of gross anatomy, our approach to the demonstration and description of cardiac structure is the same as that used by Leonardo 4 centuries ago. We now have the advantage of correlating our gross morphologic findings with the arrangements as shown during life using recently developed imaging techniques. By taking note of the innovations achieved by those working in the clinical arena, morphologists can match their innovations, since as yet examination of the autopsied specimen is still taken as the gold standard for the understanding of cardiac structure. The morphologists themselves, nonetheless, can introduce their own innovations. This is achieved by modifying, when justified, their descriptions on the basis of new interpretations of the cardiac building blocks, recognising for example the distinction of the sleeve of free-standing muscular subpulmonary infundibulum that lifts the pulmonary valvar leaflets away from the base of the ventricular mass. The ultimate goal of the categorisation of congenitally malformed hearts could well be the distinction of genotypes. If we are to move towards genotypic classification, we must first agree on how to distinguish the phenotypic variation in the various combinations encountered in patients with congenitally malformed hearts. In this light, it is surprising that, as yet, there is no consensus as how best to describe the commonest of defects, namely holes between the ventricles. As we have sought to show, this reflects the fact that those producing previous categorisations have concentrated on different morphological features of the malformed hearts so as to produce the primary criterion when making their heirarchies. Because of this, it is not even possible to produce unity from mapped codes, since we cannot be sure that the phenotypes being mapped one to the other are identical. In this review, therefore, we have sought to collate the features spotlighted in different categorisations, identifying their similarities, but also emphasising their differences. Such analysis might, hopefully, lead to a system for diagnosis and description acceptable to all schools of thought, which would surely represent a much-needed morphological innovation.



























