INTRODUCTION
Nasopharyngeal colonization by bacterial pathogens is a primary stage for the development of upper respiratory tract infection (URTI) of young children due to Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Staphylococcus aureus [Reference Wertheim1–Reference Bogaert3]. The well-known risk factors that promote carriage and spread of these pathogens in children include host immunity, overcrowding (e.g. daycare centre attendance), exposure in the family, number of siblings, passive smoking, age, and female gender [Reference Principi4, Reference Garcia-Rodriguez and Martinez5].
The adenoids play a crucial role in the aetiology of otitis media, rhinosinusitis and adenotonsillitis and knowledge of their microbial flora in individual patients is important for their treatment. Several previous studies have explored the range of bacteria colonizing the adenoids and both positive and negative associations between species colonizing the nasopharynx and adenoids have been reported [Reference Brook, Shah and Jackson6–Reference Murphy, Bakaletz and Smeesters8], but with contradictory results. There are also some data concerning significance of nasopharyngeal samples to predict acute or recurrent otitis media [Reference Karlidag9] or to corroborate a diagnosis of pharyngotonsillitis [Reference Gunnarsson, Holm and Soderstrom10] but data correlating nasopharyngeal and adenoid bacterial flora in children with recurrent respiratory tract infections are lacking.
In this study we investigated the differences and correlations in occurrence and associations of the main pathogens colonizing the upper respiratory tract (S. pneumoniae, H. influenzae, M. catarrhalis and S. aureus) in the nasopharynx and adenoids in preschool children (aged 2–5 years) who underwent adenoidectomy for recurrent URTIs. Risk factors for such colonization were also investigated in this patient cohort.
METHODS
Patients
The study enrolled 103 children, aged between 2 and 5 years, undergoing adenoidectomy in the Department of Paediatric Otolaryngology, Phoniatry and Audiology, Medical University of Lublin, Poland during May–June and November–December 2011. The indication for adenoidectomy was recurrent URTIs for at least 2 years with ⩾5 acute episodes per year. Patients did not receive antibiotic therapy for at least 20 days before the operation. In each case, informed consent was obtained from the parents and the study protocol was approved by the Ethical Committee of the Medical University of Lublin (no. KE-0254/75/211).
Demographic data of the studied children is shown in Table 1. All patients had received antibiotics during the 2–3 months prior to surgery but none had been immunized with a pneumococcal vaccine; 25 children were vaccinated against H. influenzae type b.
Table 1. Demographic data of children undergoing adenoidectomy for recurrent upper respiratory tract infections
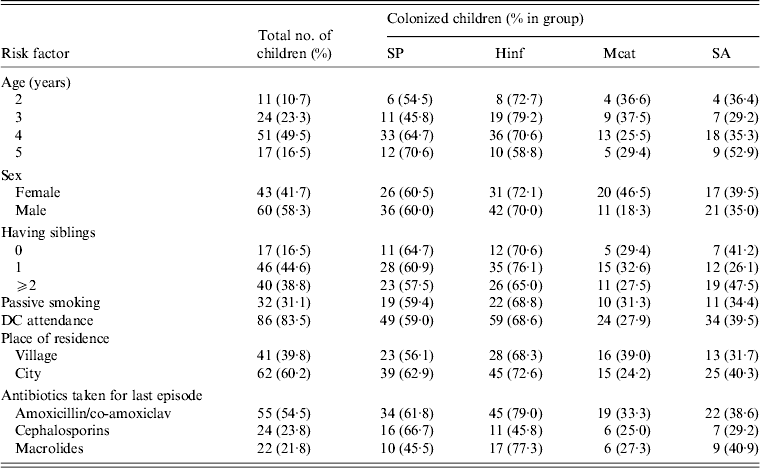
DC, Daycare centre; SP, Streptococcus pneumoniae; Hinf, Haemophilus influenzae; Mcat, Moraxella catarrhalis; SA, Staphylococcus aureus.
Laboratory procedures
Before adenoidectomy, under general anaesthesia, nasopharyngeal specimens were obtained through the nostril with sterile alginate-tipped swabs on an aluminium shaft. The surgeon removed the adenoids, under the control of an endoscope, via the mouth by making several small incisions and cauterized the site once the adenoids are removed. Antiseptic and/or bactericidal agents were not used during the surgery. The adenoids were placed in a sterile container and transported together with the nasopharyngeal swabs within 2 h to the laboratory. One surface of adenoid was cauterized with a heated scalpel and an incision was made through that cauterized area with a sterile scalpel cutting the adenoid in half. The core was swabbed with a sterile alginate-tipped applicator and swabs were inoculated on Mueller–Hinton agar with 5% sheep blood with and without 0·5 mg/l of gentamicin for selective cultivation of pneumococci, Haemophilus chocolate agar (bioMérieux, France), mannitol salt agar (bioMérieux) for selective cultivation of S. aureus, McConkey agar (Biocorp, Poland) for selective cultivation of Gram-negative rods and Sabourand agar (Biocorp) for selective cultivation of yeasts. The plates were incubated aerobically at 35°C or in 5% CO2 enriched atmosphere for 24–48 h. Pneumococci were identified by colony morphology, susceptibility to optochin (5 μg), and bile solubility and confirmed by a slide agglutination test (Slidex Pneumo-kit, bioMérieux). M. catarrhalis and H. influenzae were identified by macroscopic, microscopic and biochemical assays by API NH microtest (bioMérieux). Isolates of S. aureus were identified by colony morphology, biochemical activities (ID32 STAPH, bioMérieux), coagulase test and a slide agglutination test (Slidex Staph-kit, bioMérieux), β-haemolytic streptococci were identified by susceptibility to bacitracin (0·8 U/disc) (bioMérieux) and agglutination test for the grouping of streptococci (Slidex Strepto Plus, bioMérieux). The identification of Gram-negative rod isolates was determined using an oxidase test (Taxo N; Becton, Dickinson, USA) and Api 20E or Api 20NE (bioMérieux) as appropriate. Yeasts were identified by colony morphology, Gram staining and biochemical method (API Candida, bioMérieux).
Definitions
Nasopharyngeal culture was representative for nasopharynx microflora including microflora of adenoid surface. Adenoid culture pertained to microflora of adenoid core.
Statistical analysis
Data processing and analysis were performed using Statistica v. 10 (StatSoft Inc., USA). Potential predictor variables were tested by separate univariate analyses (χ 2 or Fisher's exact test, as appropriate) for bacterial association with upper respiratory colonization. Relative risks (RR) and their 95% confidence intervals (CI) were calculated. Logistic regression models were fitted to identify risk factors associated with bacterial carriage in nasopharynx and adenoids, separately. From these models, adjusted odds ratios (OR) and 95% confidence intervals were derived; corresponding P values were from Wald's test. Goodness of fit was checked using Hosmer & Lemeshow's test. Variables of particular interest based on previous studies, such as age, having siblings, passive smoking and type of antibiotic used, were included even when not statistically significant. Statistical significance was set at P < 0·05. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated for nasopharyngeal culture in relation to adenoid samples.
RESULTS
Bacterial carriage of targeted pathogens
In total, 103 children aged 2–5 years undergoing adenoidectomy were enrolled in the study (Table 1), and 206 paired nasopharyngeal and adenoid samples were analysed. The overall carriage rates of the targeted pathogens in both anatomical sites were for S. pneumoniae (64·1%, n = 66), H. influenzae (70·9%, n = 73), M. catarrhalis (30·1%, n = 31) and S. aureus (36·9%, n = 38). In addition, in 11 children group A streptococci were detected (two children were colonized in nasopharynx and adenoids and nine children in adenoids only). In nine children other pathogens were isolated, namely group G streptococci (three isolates from adenoid samples), one isolate each of group C streptococci, Escherichia coli, and Pseudomonas aeruginosa from adenoid samples, Acinetobacter baumanii (one isolate from a nasopharyngeal swab) and Candida albicans (two isolates from adenoid samples).
There were statistically significant differences in the frequency of bacterial isolation from both sample sites. H. influenzae was the main pathogen recovered from adenoids compared to S. pneumoniae [P = 0·001, RR 1·5, 95% CI 1·2–1·9], M. catarrhalis (P < 0·0001, RR 4·1, 95% CI 2·6–6·3) and S. aureus (P < 0·0001, RR 2·1, 95% CI 1·6–2·9). By contrast, S. pneumoniae predominated in nasopharyngeal samples compared to H. influenzae (P < 0·0001, RR 2·1, 95% CI 1·5–3·0), M. catarrhalis (P < 0·0001, RR 2·2, 95% CI 1·6–3·1) and S. aureus (P < 0·0001, RR 2·8, 95% CI 1·9–4·1). M. catarrhalis and S. aureus, respectively, were more frequently isolated from nasopharyngeal and adenoid samples (Fig. 1).
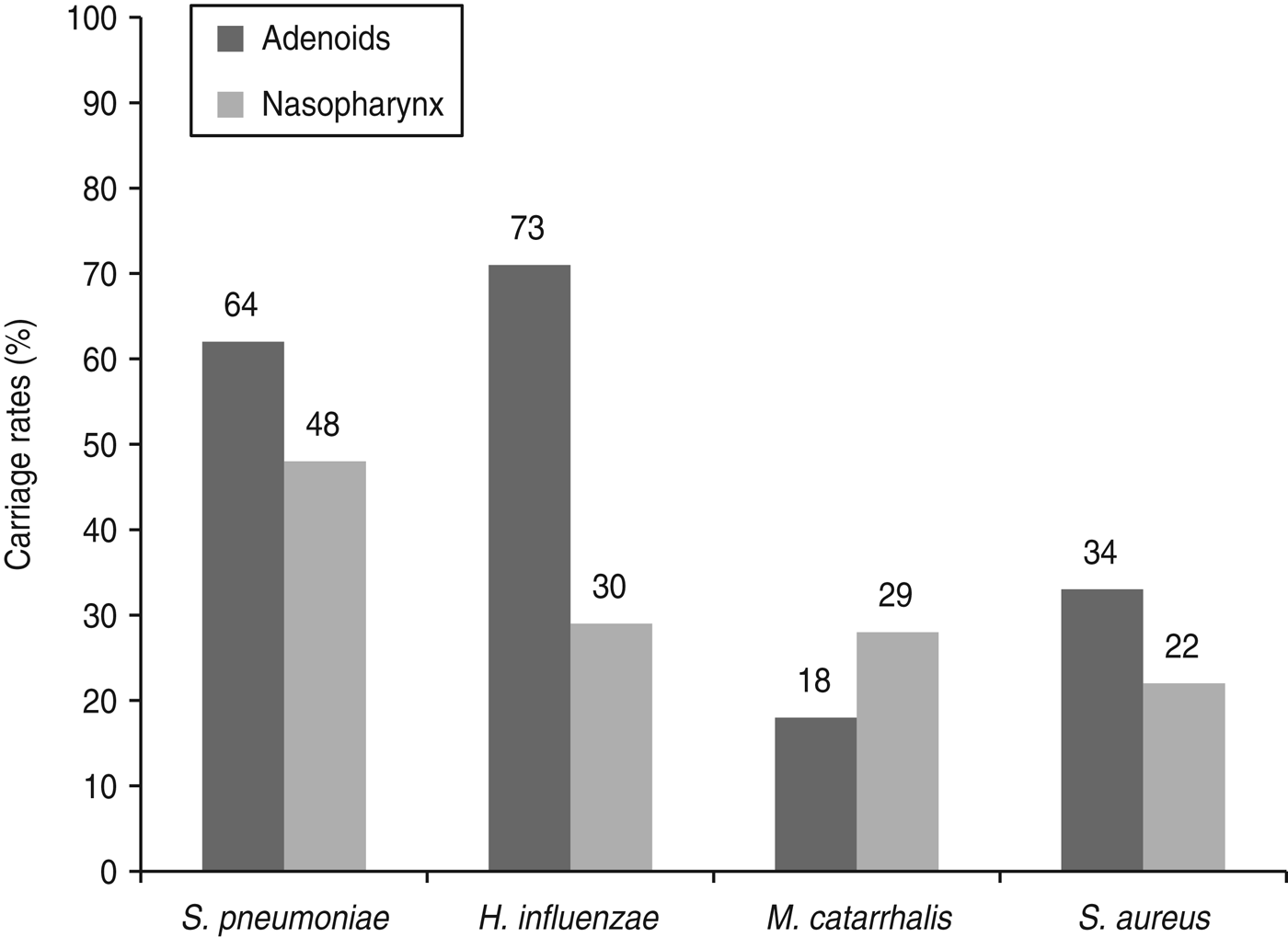
Fig. 1. Bacterial colonization according to place of isolation in children undergoing adenoidectomy for recurrent upper respiratory tract infection. Number of colonized children are shown at the top of the bars.
The distribution of the targeted pathogens isolated from both anatomical sites and their associations are presented in Table 2. Compared to adenoid samples, nasopharyngeal swabs were significantly less likely to be positive for the target species (P = 0·0001). There was a tendency for more frequent isolation of two pathogens together (40·8%, P = 0·07), and significantly, three pathogens together from adenoid samples (P = 0·02). A single pathogen was most often recovered from nasopharyngeal swabs (39·8%, P = 0·38) with M. catarrhalis being the most predominant (P = 0·003), or together with S. pneumoniae (P = 0·02). The most frequent co-colonizing species in all samples were S. pneumoniae and H. influenzae (10·7%) and this combination occurred significantly more frequently in adenoid samples (P = 0·009).
Table 2. Distribution of bacteria in adenoids and nasopharynx in children undergoing adenoidectomy for recurrent upper respiratory tract infections
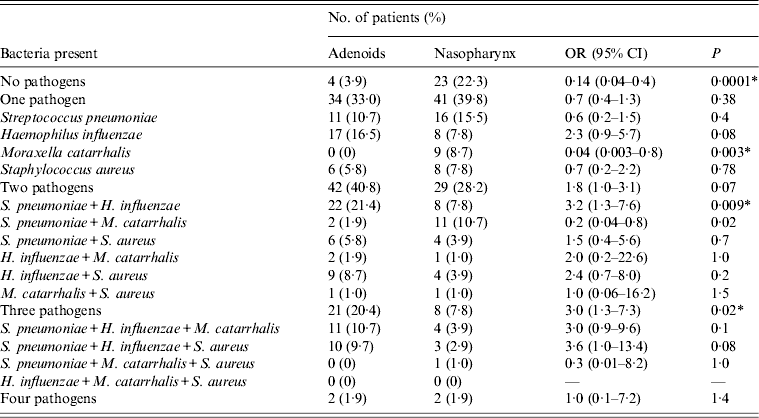
OR, Odds ratio; CI, confidence interval.
* Statistically significant.
Analysis of co-colonization, presented in Table 3, showed positive associations in adenoid samples for concomitance of S. pneumoniae and H. influenzae (P < 0·0001) compared to other species, and M. catarrhalis and S. aureus more often co-existed with S. pneumoniae and H. influenzae than with each other. No significant co-colonization was observed for nasopharyngeal swabs.
Table 3. Associations between Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Staphylococcus aureus in adenoid and nasopharyngeal samples from children undergoing adenoidectomy for recurrent upper respiratory tract infections
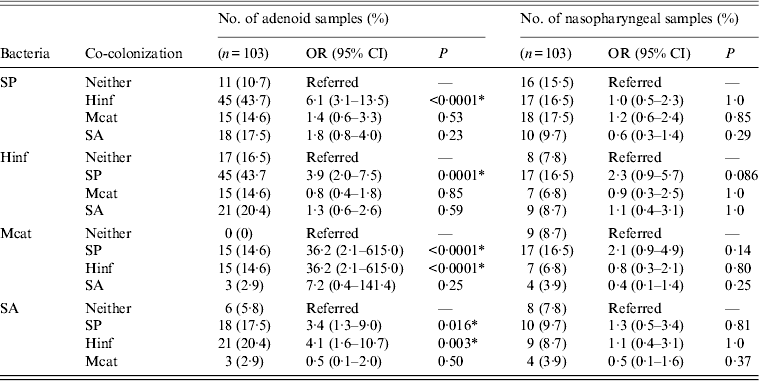
OR, Odds ratio; CI, confidence interval; SP, Streptococcus pneumoniae; Hinf, Haemophilus influenzae; Mcat, Moraxella catarrhalis; SA, Staphylococcus aureus.
* Statistically significant.
Correlation between nasopharyngeal and adenoid flora
Coherence of the same species recovered from nasopharyngeal and adenoid samples was found for S. pneumoniae, H. influenzae, M. catarrhalis and S. aureus in 47 (45·6%), 30 (29·1%), 16 (15·5%) and 19 (18·4%) children, respectively. In 17 (16·5%) children S. pneumoniae was isolated from adenoid samples only, and in two (1·9%) children it was present in nasopharynx only. H. influenzae occurred alone in adenoid samples in 43 (41·7%) children, but was never isolated alone from the nasopharynx. M. catarrhalis and S. aureus were recovered only from adenoid samples from two (1·9%) and 15 (14·6%) children, respectively, and from the nasopharynx in 13 (12·6%) and four (3·9%) children, respectively.
Analysis of sensitivity, specificity and PPVs and NPVs of nasopharyngeal colonization as indicative of the aetiology of recurrent URTIs for each pathogen showed relatively high specificity for each species (0·85–1·0); sensitivity was moderate (0·73) for S. pneumoniae, good (0·89) for M. catarrhalis and low for H. influenzae (0·41) and S. aureus (0·55). The PPVs ranged from 0·55 for M. catarrhalis to 0·96 for S. pneumoniae and 1·0 for H. influenzae, and NPVs from 0·41 for H. influenzae to 0·97 for M. catarrhalis.
Risk factors and carriage
Several statistically significant factors were associated with predisposition of subjects with nasopharyngeal and adenoid colonization to recurrent URTIs (Table 4). By multivariate analysis, female gender (P = 0·004) and city residence (P = 0·024) were identified as independent factors contributing to carriage of one or more pathogens in the nasopharynx. Correspondingly, adenoid colonization was found to be significantly associated with age (P = 0·004) and decreased by cephalosporin therapy for the most recent infection episode (P = 0·027). Rural residence significantly decreased the incidence of nasopharyngeal colonization with S. pneumoniae (P = 0·015) while this species in adenoid tissue was independently associated with age (P = 0·047). Passive smoking (P = 0·029) and not attending a daycare centre (P = 0·01) were the factors predisposing to nasopharyngeal carriage of H. influenzae. In the case of H. influenzae in adenoid samples, cephalosporins taken for the last infection episode significantly reduced its incidence. Female gender appeared to predispose to M. catarrhalis colonization in both the nasopharynx and adenoid tissue. The presence of S. aureus in the nasopharynx of subjects resident in rural areas was noted (P = 0·044), but no associated factor was evident for this organism in adenoid samples.
Table 4. Multivariate analysis of impact of epidemiological factors to carriage of Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Staphylococcus aureus in children undergoing adenoidectomy for recurrent upper respiratory tract infections
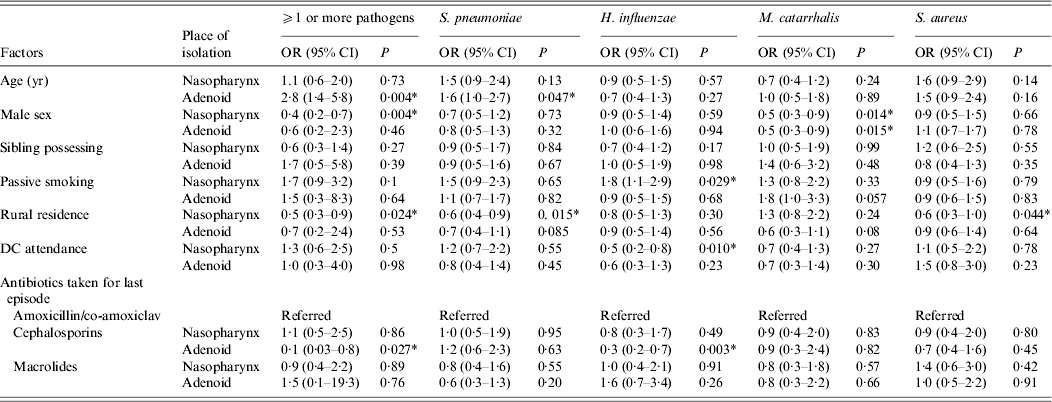
OR, Odds ratio; CI, confidence interval; DC, daycare centre.
* Statistically significant.
DISCUSSION
The most common cause of visits of paediatric patients in primary healthcare practice is pharyngotonsillitis or adenotonsillitis most often caused by viruses and group A streptococci. Treatment failure or recurrent infection is a common problem, but the presence of other agents is rarely considered. The adenoids of healthy children contain polymicrobial aerobic and anaerobic flora, including the main respiratory bacterial pathogens (i.e. S. pneumoniae, S. aureus, H. influenzae, and M. catarrhalis). Adenoids play a key role in antigen sampling and immune response induction directed against respiratory pathogens. Some organisms, however (e.g. H. influenzae [Reference Forsgren11], M. catarrhalis [Reference Heiniger12], and S. aureus [Reference Clement13]), deploy mechanisms to use pharyngeal lymphoid tissues as a niche to persist in the host. These pathogens are also highly associated with colonization of the adenoids in patients with recurrent otitis media, adenotonsillitis, and obstructive adenoid hyperplasia [Reference Brook, Shah and Jackson6]. Our study confirms a high frequency of colonization by these organisms in patients with recurrent inflammations [Reference Karlidag9].
As previously noted [Reference Brook and Shah14] we also found a high prevalence of H. influenzae in the adenoids. This species is commonly recovered from recurrent tonsillitis in children [Reference Gaffney15], in removed tonsils due to recurrent and hyperplastic tonsillitis [Reference Lindroos16, Reference Gaffney and Cafferkey17] as well as in the nasopharynx in patients with pharyngotonsillitis [Reference Soderstrom18]. H. influenzae is not clearly implicated as a cause of pharyngotonsillitis, but some studies have reported it in acute pharyngotonsillitis, sore throat, treatment failure or recurrent infections [Reference Gunnarsson, Holm and Soderstrom10, Reference Soderstrom18]. Its isolation from adenoid specimens is also reported to be significantly higher from children with otitis media with effusion [Reference Suzuki, Watanabe and Mogi19] and chronic otitis media [Reference Nistico20] than from children without these conditions. We found H. influenzae more frequently from the adenoids than from the nasopharynx, suggesting involvement with inflamed adenoids. Moreover, none of the patients showed nasopharyngeal colonization with H. influenzae alone and so its isolation would be highly predictive of the aetiology of recurrent URTI. On the other hand, the absence of H. influenzae in the nasopharynx in these children does not exclude it as a cause of adenoid infections (low NPV).
The second most frequent pathogen was S. pneumoniae with a higher detection rate in adenoid samples than the nasopharynx (62·1% vs. 47·6%). This suggests that the adenoids in children with recurrent URTIs, may be the main reservoir of S. pneumoniae where it gives rise to chronic infection, swelling and inflammation. Asymptomatic nasopharyngeal carriage of pneumococci is widely prevalent in young children and is an important factor for transmission within a community and development of pneumococcal disease; as many as 60% of healthy preschool children have been shown to carry pneumococci in the nasopharynx [Reference Garcia-Rodriguez and Martinez5]. Increased carriage rates in children have been associated with age during respiratory tract infections in the absence of otitis media [Reference Syrjänen21] but other studies have found no difference in the overall S. pneumoniae carriage rate between healthy and sick children in different age groups [Reference Greenberg22] nor have been able to confirm the species as an important causative agent of respiratory tract infection and sore throat. From our data, the presence of S. pneumoniae in the nasopharynx had high specificity and PPV as a cause of recurrent URTI.
M. catarrhalis is a human-restricted commensal organism that over the last two decades has developed into an emerging respiratory tract pathogen [Reference Murphy, Bakaletz and Smeesters8]. The species may be overlooked in culture because of its phenotypic similarity to commensal Neisseria, which are part of the normal flora of the upper respiratory tract. Currently available data on age-specific colonization rates indicate that infants are exposed to M. catarrhalis very early in life [Reference Meier23], whereas carriage in older children is lower, with reported rates of 20–54% [Reference Zemlickova24, Reference Ejlertsen25]. M. catarrhalis has been found in tonsils or in the adenoids in children with recurrent adenotonsillitis [Reference Brook and Shah14], and with recurrent otitis media [Reference Bernhard, Spaniol and Aebi2]. We found a frequency of 28% for nasopharyngeal colonization in preschool children which is similar to the report of Zemlickova et al. [Reference Zemlickova24] for the same age group (22·1%). No increased prevalence of M. catarrhalis in patients with respiratory tract infection and sore throat compared to healthy individuals has been reported [Reference Gunnarsson, Holm and Soderstrom10] which possibly suggests a benign role for the organism. In our study, M. catarrhalis was the one pathogen which was more frequently isolated from the nasopharynx than adenoid samples, which could support this statement. However, Heiniger et al. [Reference Heiniger12] demonstrated that adenoids and tonsils from patients without acute respiratory tract disease harbour a subepithelial and intracellular reservoir of M. catarrhalis which may explain the relatively poor sensitivity of adenoid surface culture. The frequency of M. catarrhalis in adenoid tissue obtained in our study (18%) was similar to that observed in adenoids in children with otitis media with effusion (11–17·1%) [Reference Karlidag9, Reference Suzuki, Watanabe and Mogi26].
The S. aureus recovery rate in our patient cohort (33%) is in accordance with other reports of nasopharyngeal carriage of 10–35% of children in whom it can on occasion give rise to respiratory infection [Reference Wertheim1, Reference Zemlickova24]. S. aureus is one of the predominant species recovered from adenoid tissue in children with recurrent otitis media (10–46·7%), recurrent adenotonsillitis (10–40%) [Reference Brook, Shah and Jackson6, Reference Brook and Shah14, Reference McCay27], and rhinosinusitis (15·6%) [Reference Shin28].
Bacterial pathogens are generally studied individually, although in their natural environment they often co-exist or compete with multiple microbial species. In this study, for the first time, relationships were compared between the four main pathogens during multiple colonization in the nasopharynx and adenoids. Multiple pathogens were found more commonly than single species in adenoids (P < 0·0001, OR 1·9, 95% CI 1·4–2·6) which is in accordance with most reports [Reference Karlidag9, Reference McCay27]. However, for nasopharyngeal colonization, there was a similar number of samples yielding single or multiple pathogens. No associations in co-colonization between species was evident in the nasopharynx whereas in adenoids several positive associations were found, most notably between S. pneumoniae and H. influenzae, and others (Table 3). Some studies have reported an inverse correlation between nasopharyngeal carriage of S. pneumoniae and S. aureus [Reference Regev-Yochay29, Reference Pettigrew30] which we also observed in our study but without statistical significance. Positive associations have been reported between pair-wise combinations of S. pneumoniae and H. influenzae and between S. pneumoniae and M. catarrhalis but not for S. pneumoniae and S. aureus, or H. influenzae and S. aureus [Reference Jacoby7]. This is supported by our data, although we found statistically significant positive associations between S. aureus and H. influenzae or S. pneumonia, but not the reverse. Xu et al. [Reference Xu31] found a synergistic association between S. pneumoniae and M. catarrhalis only in healthy children, in contrast to children with acute otitis media where competitive associations between H. influenzae and S. pneumoniae or M. catarrhalis were evident. A fundamental difference between our data and previous reports is that these studies were confined to nasopharyngeal cultures. In the adenoids, the target pathogens are localized not only on the mucosal surface but also within crypts in the tissue in aggregates of cells surrounded by a carbohydrate matrix which is characteristic of bacterial biofilms [Reference Nistico20]. Interactions between species and their virulence factors may be reduced and so it is difficult to determine the relationships, especially competitive interactions under these conditions.
Although it has been suggested that nasopharyngeal culture has significant utility for the choice of antibiotics in children with chronic adenoiditis [Reference Marzouk32], there are no data concerning the value of nasopharyngeal cultures in identification of the potential pathogens responsible for inflammation in adenoids and their predictive value for the aetiology of URTIs. In our study, these data were satisfactory only for H. influenzae and S. pneumoniae with high specificity but low sensitivity. However, our findings suggest that M. catarrhalis could be excluded as a cause of adenoiditis when not cultured from a nasopharyngeal swab due to a high NPV with good to high levels of sensitivity and specificity.
Passive smoking is associated with upper and lower tract infections, and also increases colonization by pathogens in children but the impact of tobacco exposure and carriage of pathogens is a matter of debate. Principi et al. [Reference Principi4] concluded that tobacco smoke exposure of healthy children aged <5 years did not influence pathogen carriage in the upper respiratory tract but a more recent study did show increased S. pneumoniae carriage rates in children exposed to tobacco smoke [Reference Greenberg33]. By contrast, Bakhshaee et al. [Reference Bakhshaee34] found a significant difference in carriage rates between children living in smoking families, compared to those in non-smoking families for M. catarrhalis, but not for S. pneumoniae and H. influenzae. In our cohort, passive smoking was a risk factor for increased nasopharyngeal colonization of H. influenzae and this is supported by Kosikowska et al. [Reference Kosikowska, Korona-Glowniak and Malm35] who also found increased colonization of this pathogen in healthy children aged 3–5 years, heavily exposed to tobacco smoke, as well as vulnerability to recurrent respiratory infections.
Our data suggest that place of residence of the child is a significant factor for microbial colonization, especially S. pneumoniae and S. aureus in the nasopharynx. Living in a rural area has been reported to be associated with less nasopharyngeal carriage of S. pneumoniae and H. influenzae, in children aged <5 years [Reference Principi4] but this was not supported by Boken et al. [Reference Boken36] who found no difference in the carriage rate of S. pneumoniae between rural- and urban-dwelling children. Surprisingly, in our study, children aged 4 and 5 years appear to have a higher prevalence of S. pneumoniae in the adenoids compared to younger subjects. Available data concerning age and other risk factors of pharyngeal colonization alone in children suggests that after peaking at 2–3 years of life, colonization with respiratory pathogens decreases with age [Reference Zemlickova24, Reference Mackenzie37]; the <4 years age group may be more frequently colonized by M. catarrhalis while S. aureus carriage rate is more prevalent in older children [Reference Jourdain38]. Zemlickova et al. [Reference Zemlickova24] demonstrated in healthy children aged 3–6 years that younger children (aged 3 years) were more frequently colonized by S. pneumoniae but this declined and was accompanied by a simultaneous increase in S. aureus at age 6 years. In our study, the peak incidence of S. pneumoniae and S. aureus was recorded in 5-year-olds, when the highest prevalence of H. influenzae and M. catarrhalis was at age 3 years. This difference may arise from the individuality of our studied groups, i.e. children undergoing adenoidectomy for recurrent URTIs and the specificity of the studied samples.
A significant relationship between cephalosporin treatment for the last infection and adenoid colonization by the target pathogens was evident particularly for H. influenzae. Antibiotic therapy has been cited as a significant contributor to the decrease in colonization rate of S. pneumoniae alone or in combination with M. catarrhalis in children with UTRIs, but not of H. influenzae [Reference Pettigrew30, Reference van den Bergh39]. However, antimicrobial therapy has also been implicated in a decrease of each of these species in another study [Reference Varon40].
In conclusion, we have shown that adenoids can act as a reservoir of respiratory bacterial pathogens, especially H. influenzae and S. pneumoniae, in children undergoing adenoidectomy for recurrent URTIs. However, the colonizing flora of adenoids and nasopharynx in the same patient are not always comparable. Nevertheless, the recovery of H. influenzae and S. pneumoniae in the nasopharynx could be predictive for the aetiology of URTIs. Prospective clinical trials are needed to determine the impact of adenoidectomy on composition of nasopharyngeal bacterial flora after surgery and its preventive role in possible future URTIs (i.e. acute otitis media, pharyngotonsillitis, rhinosinusitis) in young children.
DECLARATION OF INTEREST
None.








