Infants undergoing cardiothoracic surgery often experience moderate to severe pain. Appropriate post-operative pain management after cardiac surgery in children can improve both short- and long-term outcomes. In the paediatric cardiac ICU, opioids are oftentimes the medication of choice for post-operative pain. Recently, there has been a push to reduce opiate use in patients in the ICU due to the risk of adverse events like respiratory depression, sedation, nausea, vomiting, and ICU delirium. Reference Martyn, Mao and Bittner1 Alternative agents including non-steroidal anti-inflammatory drugs can be used for post-operative pain without the adverse events seen with opioids. Ketorolac, a potent non-steroidal anti-inflammatory drug, has been used successfully in adults for post-operative pain control without significant adverse events. Reference De Oliveira, Agarwal and Benzon2–Reference Lee, Cooper and Craig4 Use of ketorolac in infants after cardiac surgery has the potential to improve pain control which can lead to reduced opioid administration.
A Cochrane review demonstrated insufficient data for the efficacy and safety of ketorolac in children. Reference McNicol, Rowe and Cooper5 There is even more limited data for the use of ketorolac in infants <6 months of age. The evidence is mixed, with some small studies suggesting an increased risk of bleeding and acute kidney injury, whereas another study showed no increased risk of bleeding. Reference Aldrink, Ma and Wang6–Reference Gupta, Daggett and Drant8 Additionally, there is limited data if ketorolac is effective at post-operative pain control in paediatric cardiothoracic patients.
At our institution, an analgosedation protocol was implemented utilising ketorolac in infants who underwent cardiothoracic surgery. Patients following this protocol would receive ketorolac IV 0.5 mg/kg (max 30 mg) every 6 hours for a total of eight doses. The aim of this study is to evaluate the safety and efficacy of ketorolac in this patient population.
Methods
This study is a single-centre retrospective study conducted at a paediatric tertiary care facility, including infants undergoing cardiothoracic surgery between 09/01/2017 and 08/31/2019. In August 2018, an analgosedation protocol was implemented which included the use of post-operative IV ketorolac. Patients were eligible to be included in the study if they received or did not receive ketorolac based on respective study group, were admitted to the cardiac ICU, were greater than 37 weeks gestational age, were greater than 30 days old and less than 6 months, were planned post-operative intubation <48 hours, platelets > 100,000/uL, and did not meet acute kidney injury definition per Kidney Disease Improving Global Outcomes (KDIGO) criteria. Patients were divided into two cohorts: 12 months pre-protocol versus 12 months post-protocol. The primary outcome was to determine the efficacy of ketorolac in terms of total opiate use in morphine milligram equivalents reduction for 72 hours post-operatively.
Secondary outcomes evaluating the safety of ketorolac included the incidence of acute kidney injury and rate of bleeding complications. Acute kidney injury was defined as meeting serum creatinine criteria for stage 1 or greater as defined by KDIGO. 9 Post-operative bleeding complications were defined as the amount of drainage from chest tubes (mL/kg/day) and need for transfusion.
Pre-protocol analgosedation utilised a similar regimen as the protocol with the exception of scheduled ketorolac and acetaminophen. Pre-protocol patients received continuous dexmedetomidine, intermittent intravenous morphine, and/or an oral opioid (oxycodone, hydrocodone/acetaminophen) with nursing parameters to maintain face, legs, activity, cry, and consolability (FLACC) score < 4 and/or state behavioural scale score 0 – −1. Routine intermittent opioid doses were used in both cohorts to include morphine 0.05–0.1 mg/kg/dose q2h, oxycodone 0.05–0.01 mg/kg/dose q6h, and hydrocodone/acetaminophen 0.05 mg/kg/dose q6h. Continuous infusion opioids were given at the bedside providers discretion. Acetaminophen was usually scheduled for the first 24–48 hours post-operatively but was ordered at provider discretion. The post-protocol group received continuous dexmedetomidine, scheduled enteral acetaminophen for 24 hours, intermittent intravenous morphine with nursing parameters to maintain FLACC score < 4 and/or state behavioural scale score 0 – −1, and ketorolac 0.5 mg/kg every 6 hours for 48 hours post-operatively (Supplementary Material). Continuous infusion opioids were given at the bedside providers discretion. Oral non-steroidal anti-inflammatory drugs, anxiolytics, g gama-aminobutyric acid (GABA) modulators, and additional multimodal agents were not routinely used in either group. There was no change in intraoperative analgesic or sedation medication administration during the entirety of the study period.
Descriptive statistics were summarised as medians and interquartile ranges, and frequencies and percentages. Wilcoxon rank-sum tests were used to analyse continuous variables. Chi-square tests or Fisher’s exact tests were used for all categorical variables. Linear mixed effects models with unstructured covariance structure were fit to evaluate the effects of treatment on morphine milligram equivalents over different time points (0–24 hours, 24–48 hours, and 48–72 hours) and on serum creatinine before and after surgery. Since the residuals for morphine milligram equivalents were skewed, an inverse hyperbolic sine transformations were applied to normalise the residuals. Means were back-transformed for interpretation of results on their original scale. Least square (LS) means and their respective 95% confidence Intervals are presented for serum creatinine and bleeding outcomes. A difference-in-difference analysis was performed to determine the mean change in serum creatinine levels from pre-surgery to post-surgery in ketorolac group compared to the control. Statistical significance was significant at p < 0.05. All analyses were performed in SAS v9.4 (Cary, NC). This study was conducted under an Institutional Review Board protocol approved by Children’s Healthcare of Atlanta (STUDY00001287; Evaluating the Safety of Ketorolac in Infants <6 months after Congenital Heart Surgery; approved December 21, 2021), and the need for written consent was waived.
Results
A total of 243 patients were identified from the study period and reviewed. Of those, 98 patients were excluded from the study. Sixty-nine patients were excluded as they received ketorolac prior to the initiation of the analgosedation protocol and 29 patients were excluded as they did not receive ketorolac post-protocol implementation. A total of 145 patients were included for final analysis. Fifty-seven patients did not receive ketorolac (pre-protocol group) and 88 patients received ketorolac (post-protocol group). The baseline demographics between the groups were similar (Table 1).
Table 1. Baseline demographics
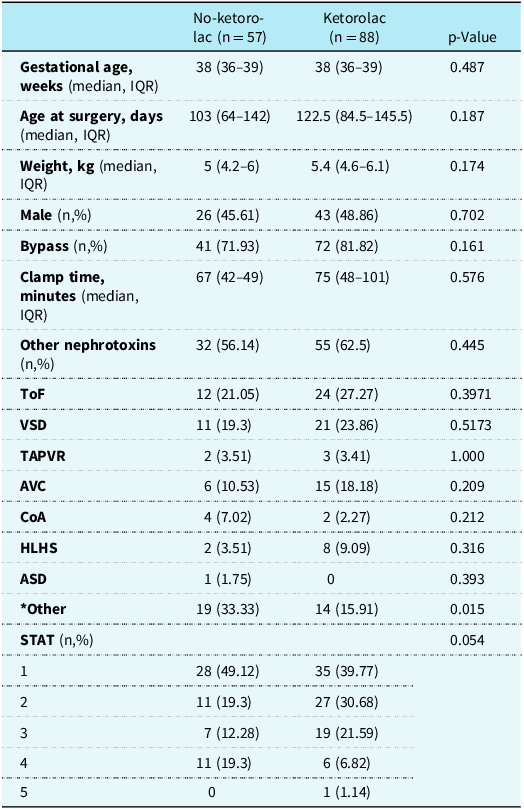
ASD = atrial septal defect; CoA = coarctation of the aorta; HLHS = hypoplastic left heart syndrome; STAT = the Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery; TAPVR = total anomalous pulmonary venous return; ToF = tetralogy of Fallot; VSD = ventricular septal defect.
* Other: pulmonary atresia, pulmonary valve stenosis, vascular ring, patent ductus arteriosus, mitral atresia, double outlet right ventricle, double-inlet left ventricle, pericardial effusion, sternal wire removal, exploration, and chest closure
Patients administered ketorolac used less cumulative opiates, in morphine milligram equivalents, POD 1–3 than patients not receiving ketorolac (9.47 versus 12.68; p = 0.002) (Table 2). Additionally, when further evaluated by POD, the no-ketorolac group required more opiates on POD 1 (10.9 versus 5; p < 0.001) and POD 2 (4.2 versus 2.5; p = 0.006) with no difference found on POD 3 (2 versus 1.6; p = 0.2) (Fig 1). There was no difference in the percentage of patients who received a continuous opioid infusion within the first 72 hours on POD 1–3 between the no-ketorolac group and ketorolac group (33% versus 23%; p = 0.2).
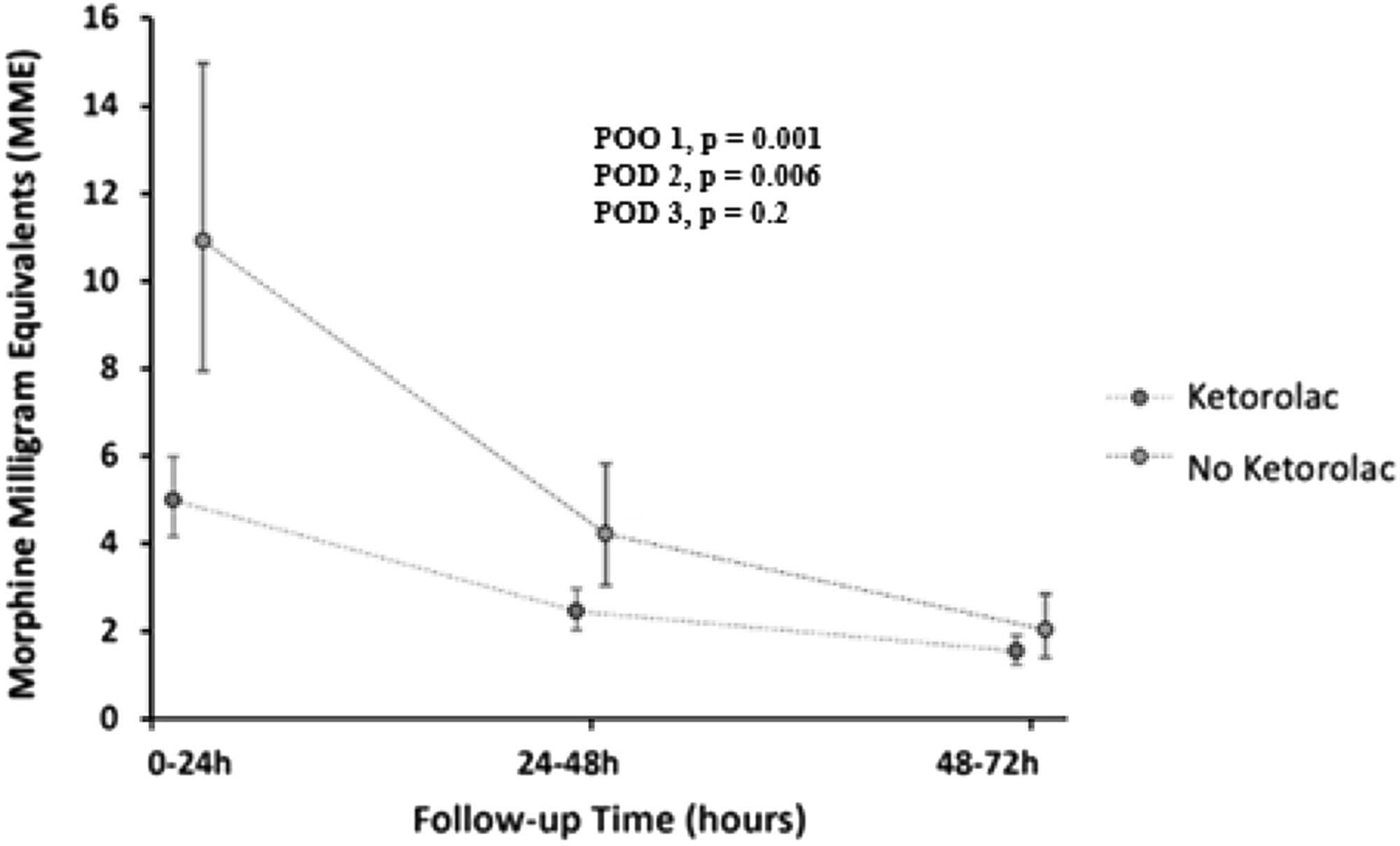
Figure 1. Comparing the use of opiates in morphine milligram equivalents in the first 72 hours post-op in patients receiving and not receiving ketorolac.
Table 2. Use of opiates in morphine milligram equivalents post-op days 1–3
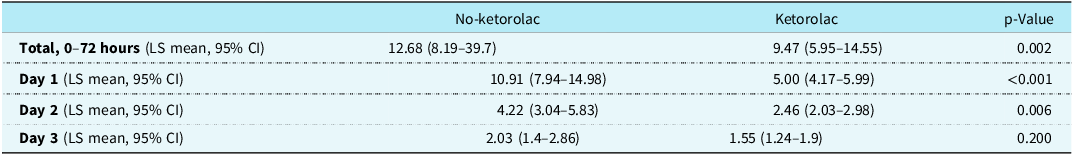
There was a significantly higher incidence of stage 3 acute kidney injury in the no-ketorolac group (7.02% versus 0%; p = 0.022); however, there was no difference in stage 1 (28.07% versus 22.73%; p = 0.623) and stage 2 (28.07% versus 15.91%; p = 0.077) acute kidney injury between groups. After adjusting for gestational age, age at surgery, STAT (The Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery) score, whether or not they were on a nephrotoxic agent, whether or not they were on bypass, and clamp time, we found that the mean baseline serum creatinine [0.25 (0.23–0.27) versus 0.29 (0.27–0.31); p = 0.046] was higher in the ketorolac group. However, the mean highest recorded serum creatinine value on POD 1–3 [0.4 (0.37–0.43) versus 0.38 (0.36–0.4); p = 0.224] was higher in the no-ketorolac group. There was a higher mean increase from baseline to highest post-operative serum creatinine on POD 1–3 in the no-ketorolac group compared to the ketorolac group (0.15 versus 0.09; p = 0.014). There were no differences in average chest tube output in mL/kg/day (0.24 versus 0.32; p = 0.569) or need for transfusion (36% versus 24%; p = 0.125) between no-ketorolac and ketorolac, respectively.
Discussion
Based on the findings in our study, the use of ketorolac is likely safe and effective in infants greater than 30 days and less than 6 months of age after cardiothoracic surgery. McElroy et al evaluated the use of ketorolac in infants less than 6 months of age after surgery and found similar results. Reference McElroy, Bustin and Gattoline10 They concluded that ketorolac appears to have a low incidence of major bleeding without renal and/or coagulation dysfunction. Similarly, Moffett et al states that ketorolac is safe to use in neonates and infants after cardiac surgery as ketorolac was not associated with any adverse haematologic or renal effects. Reference Moffett, Wann and Carberry11
To our knowledge, this is the only study that evaluated opioid exposure in paediatric cardiac patients receiving ketorolac. Infants receiving ketorolac were administered significantly less opioids than patients not receiving ketorolac. Patients who received ketorolac were administered 0.5 mg/kg of IV ketorolac every 6 hours for up to eight doses starting 12 hours post-op. Similar sedation and analgesic regimens were utilised in both groups to include intraoperative management. Patients in the ketorolac group did receive scheduled acetaminophen for the first 24 hours; however, it was common practice to schedule acetaminophen in the pre-protocol group for 24–48 hours despite the practice not being protocolised to a computer physician order entry order set. Patients not receiving ketorolac were administered 2.2 times more opioids on POD 1 and 1.7 times more opioids on POD 2 than patients receiving ketorolac. On POD 3, patients received 1.3 times more opioids in the no-ketorolac group compared to the ketorolac group; however, this was not a statistically significant finding.
Two serum creatinine values were measured. The first serum creatinine level was defined as the last level taken prior to surgery (pre-op), while the second level was the highest serum creatinine value obtained up to 72 hours post-operatively (i.e., POD 1–3). Of note, a small change in serum creatinine can classify a patient as having an acute kidney injury as defined by KDIGO, although the increase in serum creatinine may not be clinically significant. Multiple variables deemed to potentially effect serum creatinine were collected to include if patients were on concurrent nephrotoxic agents as dictated by the NINJA (nephrotoxic injury negated by just-in-time action) initiative, gestational age, age at surgery, STAT, need for cardiopulmonary bypass, and aorta clamp time. Reference Goldstein, Mottes and Simpson12 After adjusting for these variables, our study showed that patients receiving ketorolac did not have a higher incidence of acute kidney injury or mean increase in serum creatinine after surgery compared to the ketorolac group. This demonstrates that there was not an increased risk of acute kidney injury in patients receiving ketorolac after congenital heart surgery.
The use of ketorolac did not result in an increase in bleeding events. Bleeding was assessed by the average amount of chest tube output per day in mL/kg/day. Bercovitz et al published an article which aimed to validate an objective definition of post-operative bleeding in infants and neonates post-cardiac surgery. Reference Bercovitz, Shewmake and Newman13 This study evaluated average chest tube output in mL/kg/h over different post-operative periods in order to determine if excessive bleeding occurred. Although, it cannot be assumed that all chest tube output is bloody, it is possible to infer that increased chest tube output is correlated with increased post-operative bleeding. In our study, we found no difference in the quantity of chest tube output in the ketorolac and no-ketorolac group. This illustrates that the use of ketorolac is likely safe to use in infants after congenital heart surgery.
Our findings are subject to all limitations inherent to single-centre retrospective cohort studies. Pain and sedation are difficult to effectively evaluate in the paediatric population due to limited language, inability to comprehend pain perception, and need for specialist pain assessment tools. Post-operative bleeding was difficult to quantify as there was inadequate documentation regarding type of fluid removed from the chest tube. Ketorolac is reported to cause platelet dysfunction, and platelets were not evaluated outside of the initial inclusion criteria and need for platelet transfusions as a secondary outcome. The exclusion criteria we used in this study was based on the exclusion criteria in the analgosedation protocol. However, there were some patients who were placed on the analgosedation protocol that should have been excluded. The number of patients who received all eight ketorolac doses was not recorded; however, typically the only reason providers would deviate from the post-operative order set with regard to ketorolac discontinuation is if a patient developed acute kidney injury or bleeding. Overall compliance of the anaglosedation protocol was not quantified. Given there was a low rate of acute kidney injury and bleeding in the patients receiving ketorolac, it is assumed that the majority of patients received all eight scheduled ketorolac doses. Time intubated was not quantified and compared amongst the two cohorts; however, all patients included had similar baseline demographics and were planned to be extubated with 48 hours of cardiothoracic surgery. Additionally, by excluding patients who had baseline abnormalities in renal function, it is possible we could have created an exclusion bias. The analgosedation protocol was established to encourage a decrease in opioid use, which could have confounded our results. Lastly, patients on the analgosedation protocol did not receive ketorolac until 12 hours post-surgery; therefore, some of the post-operative serum creatinine levels were recorded prior to the first ketorolac dose.
Conclusion
Our study showed that ketorolac use in infants after cardiothoracic surgery resulted in a significant reduction in opioid exposure. Additionally, there was not an associated increased risk of acute kidney injury or bleeding. Therefore, the use of ketorolac in infants greater than 30 days and less than 6 months of age after congenital heart surgery is likely safe and effective. Our findings are subject to all limitations inherent to single-centre retrospective cohort studies.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S104795112400091X.
Acknowledgements
None.
Financial support
None.
Competing interests
None.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and have been approved by the institutional committees at Children’s Healthcare of Atlanta.






