Introduction
Livestock system relies heavily on nitrogen (N) as a crucial nutrient for production (i.e., meat, milk, etc.). However, a significant portion, approximately 70%, of the N from feed consumed by dairy cows is excreted as urine and faeces, rather than being converted into saleable products (Yan et al., Reference Yan, Frost, Agnew, Binnie and Mayne2006; Kebreab et al., Reference Kebreab, Strathe, Dijkstra, Mills, Reynolds, Crompton, Yan and France2010; Cheng et al., Reference Cheng, Sheahan, Gibbs, Rius, Kay, Meier, Edwards, Dewhurst and Roche2013). This excretion, when urine encounters faeces, leads to the production of ammonia (NH3) gas through the reaction of urinary urea with the urease enzyme present in faeces. Livestock, particularly cows, contribute up to 40% of NH3 emissions of agricultural origin, primarily originating from manure (Chen et al., Reference Chen, Sun, Bai, Dassanayake, Denmead and Hill2015; Brandani et al., Reference Brandani, Lee, Auvermann, Parker, Casey, Crosman, Gouvêa, Beck, Bush, Koziel and Shaw2023). Such emissions pose environmental threats, causing air and water pollution (Bouwman et al., Reference Bouwman, Van Vuuren, Derwent and Posch2002; Todd et al., Reference Todd, Cole and Clark2006), and negatively impacting ecosystems and public health (WHO, 2021). The volatilized NH3 can further contribute to the formation of nitrous oxide (N2O), a potent greenhouse gas (Chen et al., Reference Chen, Sun, Bai, Dassanayake, Denmead and Hill2015), resulting in nutrient loss and reduced manure value as a fertilizer source, affecting dairy cow productivity and farm profitability (Stott and Gourley, Reference Stott and Gourley2016). Consequently, accurate quantification and research into strategies for mitigating NH3 emissions are crucial for sustainable livestock production.
Quantifying NH3 emissions is challenging due to its rapid reactivity in the atmosphere. Direct quantification methods involve chemical reactions and include acid traps, detector tubes, electrochemical sensors, passive gas absorption, denuders, optical absorption techniques and chemiluminescence techniques (Hristov et al., Reference Hristov, Hanigan, Cole, Todd, McAllister, Ndegwa and Rotz2011). However, the application of these methods faces challenges at larger scales due to logistical complexities, geographic requirements and high labour and maintenance costs. Therefore, indirect methods, such as biomarkers (manure N: phosphorus [P] ratio, N: potassium [K] ratio, and N isotopic fractionation [δ 15N]), offer alternatives (Hristov et al., Reference Hristov, Zaman, Vander Pol, Ndegwa, Campbell and Silva2009). However, these emerging indirect, biomarker-based methods were not explored widely under diverse manure production conditions.
Thus, this experiment aimed to add value to the literature by testing the hypothesis that changes in manure δ 15N, N: P, and N: K can serve as indicators for estimating NH3 emissions, with a wide range of NH3 emissions generated from manipulation of manure N composition and application of lignite as a known NH3 mitigation product.
Material and Methods
The experiment was approved by the Animal Ethics Committee from the Faculty of Science, The University of Melbourne, Australia (No. 1914778.1).
Farm location, milking and feeding system
The experiment was conducted at Dookie dairy farm (−36.4°S, 145.7°E. and 140 m above sea level). The farm has a total area of 126 ha and operates a grazing/feed pad feeding system. The farm has an Automatic Milking System which has a capacity of milking 180 mixed-age dairy cattle (AMS; Lely Astronaut; Lely, Maassluis, The Netherlands), coupled with an automatic weighing and pellet feeding system. A total of 20 lactating cattle (average live weight = 608.1 ± 69.64 kg; average lactation day = 207.8 ± 118.53 d; mean ± sd) were selected from the mixed age Holstein Friesians cattle herd at 9:00 am on 15th August 2019.
Feed sampling and nutritive value analysis
The feed samples were collected at 9:00 am daily from the farm between 12th and 14th of August 2019, three days prior to the start of urine and faeces samplings. The quantity of pellets offered, refused, and consumed by each dairy cattle per time when they visited the robots was recorded by AMS. The pellets were directly sampled through AMS. Barley hay was sampled by taking one core sample from each bale used during the experimental period. A total of 15 fresh oat crop samples (cut full height above ground) were randomly sampled from the paddock on 12th August 2019. The same method was used to sample ryegrass-based pasture on the 13th and 14th of August 2019. The collected feed samples were stored at −20°C in the Dookie Campus Science Laboratory. All frozen feed samples were then bulked per sample type and directly sent with an icebag to the New South Wales Department of Primary Industries (NSW DPI), Australia, for analysing DM, crude protein, DM digestibility (DMD), digestible organic matter in DM and estimated metabolizable energy (ME) using a Near-Infrared Reflectance Spectroscopy (NIR systems model 6500, Foss, Hillerød, Denmark).
Metabolizable energy measurement and diet intake estimation
The ME required for cattle maintenance (MEmaintenance), activity (MEactivity), lactation (MElactation), diet ME intake (MEdiet intake), diet DM intake (DMI) and ME concentration of the diet (MEdiet) were calculated following the published equations (Eqns (1)–(6)) following Nicol and Brookes (Reference Nicol and Brookes2007):
where Hkm was horizontal walking distance (km/d) and Vkm was vertical climbed distance (km/d) that were estimated from google map distance with identified daily grazing zone location; K m = MEdiet (MJ/kg DM) × 0.02 + 0.5.
where NE (MJ NE/kg) = 0.376 × Fat concentration of milk + 0.209 × Protein concentration of milk + 0.976; k is the efficiency with which ME is utilized, k = MEdiet (MJ/kg DM) × 0.02 + 0.4.
The fat and protein analyses were performed by infrared spectroscopy method.


where grazing intake (kg/d) = Oat crops intake (kg/d) for day 1; Pasture intake (kg/d) for days 2 and 3.
The ME for grazing intake (oat crops for day 1 and pasture for days 2 and 3), pellets intake, and barley hay intake were calculated by following equations (Eqns (7)–(9)):



Besides,

The grazing intake could be calculated using (Eqns (1) to (10)), then MEactivity, MElactation, MEdiet intake, MEgrazing intake, DMI and MEdiet could be calculated based on grazing intake.
Urine and faeces sampling and analysis
A 300 ml sample of urine was collected from each dairy cattle, following vulva stimulation for a maximum of 5 min. If urination did not occur within 5 min, the dairy cattle were released, and sample collection was attempted with the next dairy cattle in the queue. A total of 6 l urine from 20 cattle was collected and well-mixed in a bucket. To reduce NH3 emissions from urine samples, a container with ice was placed below the urine collection bucket. After each urine sampling, 300 g of faeces sample was collected from rectal grab. A total of 6 kg of faeces samples from 20 cattle was collected and mixed in a bucket.
For UN% and FN% analysis, two 5 ml of well-mixed urine samples and one 5 g of faeces sample were subsampled immediately into 15 ml test tubes, and one drop of concentrated H2SO4 (98%) was added into each tube for sample acidification (with a targeted sample pH < 4). All samples were stored in a freezer at −20°C at Dookie Campus Science Laboratory. One urine and one faeces subsample were pre-processed and analysed via Dumas Combustion on a LECO Trumac CN (TruMac CNS Macro Analyzer, LECO Corporation, Australia) at a furnace temperature of 1250°C. Results of UN% and FN% were reported as w/w concentration. Another urine subsample was analysed for creatinine concentration (Cobas Integra 400 plus, Roche Diagnostics, Australia).
Nitrogen balance calculation
The N balance (NB) was calculated by the following equations (Eqn. (11)) according to Spanghero and Kowalski (Reference Spanghero and Kowalski1997):
where NI was N intake, which was calculated from dietary N concentration and DMI; the UN and FN were urinary N and faecal N; the milk N, which was calculated with individual dairy cattle MY and milk protein concentration (provided by Lely robotic milking system) ÷ 6.38, 6.38 was the conversion factor of N values from milk protein values.
The NI, UN, FN and MN were calculated as following equations (Eqns (12) to (20)):



where,
where UW is the weight of urine excreted daily from dairy cattle, and


Ammonia emissions measurement using dairy cattle manure through in vitro incubation
Lignite properties and application preparation
Raw lignite (Latrobe Valley, Victoria, Australia) was ground by a pestle and mortar and then passed through a sieve (approximately 500 μm in diameter). A total of 2.1 kg of lignite powder was prepared, and one 10 g of subsample was dried in the oven (65°C) for three days to determine the DM of lignite. One 10 g of lignite subsample was sent to the University Melbourne TrACEES Platform for the analysis of total N concentration and N isotope fractionation (δ 15N) using a Thermo Flash 2000 HT (elemental analyser, Thermo Scientific, Australia) paired with a Thermo Delta V Advantage (mass spectrometer, Thermo Scientific, Australia) (CF – IRMS). The results were calculated using thermal conductivity detector (TCD) output, which was calibrated against acetanilide (Thermo Fischer, Bremen, Germany), and reported as weight/weight concentration. The analysis of lignite pH and electrical conductivity (EC) was performed based on sections 3A1 and 4A1 in the soil chemical methods book published by Rayment and Lyons (Reference Rayment and Lyons2011).
Incubation system and experimental design
Eight incubation systems were constructed at the Dookie Campus Science Laboratory, at the University of Melbourne. The incubation system was adopted from Lee et al. (Reference Lee, Hristov, Cassidy and Heyler2011) study. Each system consisted of 5 components: (1) an air pump (Stella 110, Aqua One, Australia), which propelled the air into the system; (2) an airflow meter (LZB-3WB 0.15–1.5 l/min, Darhor, China), which controlled and monitored the airflow rate; (3) a 900 ml clip water container (12.5 cm height, 13.5 cm width, and 10.5 cm length, Kmart, Australia), which was filled with water (500 ml) to provide moisture and to keep the surface of the manure from drying out during the incubation period; (4) a 2.3 l clip manure incubation container, which was made of polypropylene and silicone (16.6 cm height and 16.4 cm diameter, Kmart, Australia); and (5) an acid trap composed of a 500-ml volumetric flask (Flask Erlenmeyer 500 ml 29/30, Quickfit, England), which contained 0.5 M sulphuric acid to capture the emitted NH3 from the manure incubation container (Supplementary file). A clear vinyl tube (10 mm diameter, Pope, Australia), made of plasticized polyvinyl chloride compound, was used to connect each component of the incubation system. A universal tap adaptor (12 mm diameter, Pope, Australia) was used to secure and provide a leak-free connection between containers and tubes. A leakage test was performed for each incubation system before experimenting. A detergent (Dishwashing liquid, Earth choice, Ferntree Gully, Victoria, Australia) and water solution was applied to each connection point after the air pump was on. This was done to detect leaks, which would cause bubbles to form if present.
The incubation experiment was conducted at room temperature at the Parkville Campus, the University of Melbourne, Melbourne. The experimental design included one four treatment groups: 1 urinary nitrogen (U) to 1 faecal nitrogen (F) ratio (CT), 2 U to 1 F ratio (2U1F), and CT and 2U1F with 250 g lignite application (CT + L and 2U1F + L, respectively). The applied raw lignite was sourced from Latrobe Valley, Victoria, Australia. The incubation experiment had two trial periods, and each included two replications per treatment, with a total of four replications achieved per group for data analysis. The first period was conducted from 18th October to 2nd November 2019, and the second period was conducted from 15th November to 30th November 2019. Each period lasted for 15 days. Each incubation system contained 600 g reconstructed manure (Table 1) based on the pre-determined UN% and FN% (LECO Trumac CN method, TruMac CNS Macro Analyzer, LECO Corporation, Australia) to achieve a manure UN to FN ratio of 1 to 1 for CT and CT + L, and 2 to 1 for 2U1F and 2U1F + L. Further, 250 g lignite powder was added into CT + L and 2U1F + L and mixed with manure. To reconstruct the manure, frozen urine and faeces were thawed for 24 h at room temperature and weighed according to Table 1. They were then mixed using a glass rod for 30 s before being added to the container for incubation. The acid trap flask contained 500 ml of 0.5 M H2SO4 daily. The chemical reaction in the aid trap is expressed by following the equation (Eqn. 21):
Table 1. The input of urine, faeces, and lignite in the experiment
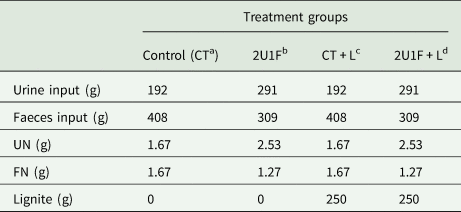
a CT used a manure with UN:FN = 1:1.
b 2U1F used a manure with UN:FN = 2:1.
c CT + L used a manure with UN:FN = 1:1 + 250 g lignite application.
d 2UIF + L used a manure with UN:FN = 2:1 + 250 g lignite application; UN:FN = urinary nitrogen: faecal nitrogen.
Sampling and analysis of fresh manure and acid trap samples
Manure samples from in vitro incubator
During each incubation period, manure samples from each treatment were collected at 10:00 once a day. For each collection, the air pump was turned off, and an incubation container was opened for less than one minute to collect manure using a calibrated plastic straw from five randomly selected spots. Five manure samples were then bulked to provide ~ 15 g of manure sample per replication daily for analysis. After the manure sample was collected, the container was closed, and the air pump was turned on to continue incubation. Each collected manure sample was freeze-dried by a freeze dryer (GAMMA 1–16 LSC plus, Martin Christ Freeze Dryer, Germany) for five days, then weighed to determine the DM. Each freeze-dried manure sample was then ball-milled using a tissue lyser (TissueLyser II, Qiagen, Germany) prior to chemical analysis. The δ 15N and total N of manure samples were analysed by the CF – IRMS. Both data were calculated using TCD output, calibrated against acetanilide (Thermo Fischer, Bremen, Germany), and reported as weight/weight concentration. Manure P and K samples were analysed using a Perkin Elmer 8300 DV ICP-OES (Syngystix v3.0, Perkin Elmer, China) relative to a 5% nitric acid sample matrix. Data were reported as weight/weight concentration.
Acid trap sample collection and analysis
A total of 15 ml of acid sample was collected daily from each incubation system and stored frozen (−20°C). The collected acid was used to determine NH3 concentration per 24-h incubation period. The distillation and titration methods were used to determine (NH4)2SO4 concentration in acid samples (Vinklárková et al., Reference Vinklárková, Chromý, Bittova, Šprongl and Žaludová2015). The concentration of (NH3) was calculated following the equation (Eqn. 22):
where 17.03 and 132.14 are the molar mass of NH3 and (NH4)2SO4 (g/mol), respectively, factor 2 is the match number from the chemical reaction.
Statistical analysis
The manure total N, P and K concentration data were corrected for the manure total N, P and K concentrations that would be from samples removed from incubation systems using daily manure total N, P and K concentration data and manure amount. The manure δ 15N and NH3 data were corrected for the δ 15N and NH3 that would be from samples removed from incubation systems using daily manure δ 15N and NH3 data and corrected manure total N.
The Genstat statistical package (16th version, VSN International group) was used to perform statistical analysis in this experiment, including general ANOVA, and linear/power/polynomial regressions. The significance of linear was determined using general ANOVA with manure composition + lignite application + day as treatment structure, and replication/treatment as block structure.
Results
Daily ammonia-nitrogen emission, cumulative ammonia-nitrogen, and manure nitrogen isotope fractionation
In the CT and 2U1F treatments (non-lignite groups), the NH3-N emission decreased over time. The curves of CT + L and 2U1F + L treatments (lignite groups) were the opposite with non-lignite groups, which increased with days. There was a weak relationship between temperature and daily NH3-N emission in four treatments (R 2 = 0.30, P ≤ 0.05; Supplementary file).
The cumulative NH3-N emission increased over time in four treatments (R 2 = 0.99, P ≤ 0.001; Supplementary file). The lignite application reduced the cumulative NH3-N emission by 87.5% compared to CT and CT + L treatments and 82.9% compared to 2U1F and 2U1F + L treatments. The NH3-N divided by manure total N was used to calculate the percentage of manure total N lost as NH3-N. It was found that 80% and 63.4% of the total N loss from manure was lost as NH3-N in the CT and 2U1F treatments, respectively.
The manure δ 15N (‰) from the CT and 2U1F treatments from 15 days of dairy manure incubation increased with incubation days (R 2 = 0.85 and 0.91, respectively; P ≤ 0.001). However, there was no relationship between manure δ 15N from the CT + L, 2U1F + L treatments and incubation day (R 2 = 0.07 and 0.08, respectively; P > 0.05).
Manure nitrogen to phosphorus ratio and manure nitrogen to potassium ratio
The manure N: P ratio (g/g) from four treatments decreased with incubation days (R 2 = 0.81, 0.73, 0.81 and 0.84 in CT, 2U1F, CT + L, and 2U1F + L treatment, respectively; P ≤ 0.001; Supplementary file). The manure N: P ratio decreased with incubation days in four treatments. In the non-lignite group, the manure N: P ratio from the 2U1F treatment was higher than it was from the CT treatment.
There was a weak relationship between manure N: K ratio and incubation days found in CT treatment (R 2 = 0.28, P ≤ 0.001; Supplementary file), while the relationships were strong in the other three treatments (R 2 = 0.70, 0.79, and 0.90 in 2U1F, CT + L, and 2U1F + L treatment, respectively; P ≤ 0.001). The manure N: K ratio decreased with incubation days in four treatments. Opposite to the manure N: P ratio, the manure N: K ratio from the CT treatment was higher than it was from the 2U1F treatment. Similar to the non-lignite group, the manure N: K ratio from the 2U1F + L treatment was lower than the CT + L treatment.
Cumulative ammonia-nitrogen emission and manure nitrogen isotope fractionation
Figure 1(a) and (b) show the relationships between cumulative NH3-N emission and manure δ 15N. In the non-lignite group, there were strong relationships between cumulative NH3-N emission and manure δ 15N (R 2 = 0.79 and 0.90 in CT and 2U1F treatment, respectively; P ≤ 0.001). Besides, the relationship between cumulative NH3-N emission and manure δ 15N in the 2U1F treatment was stronger than it was in the CT treatment. However, there was no relationship found in the lignite group (R 2 = 0.02 and 0.0003 in CT + L and 2U1F + L treatment, respectively; P ≤ 0.001). The strong relationship between cumulative NH3-N emission and manure δ 15N from the non-lignite group (combined CT and 2U1F treatments; R 2 = 0.83, P ≤ 0.001; Fig. 1(c)). However, no relationship was found when combined CT + L and 2U1F + L treatments (Lignite group; R 2 = 0.02, P ≤ 0.001; Fig. 1(d)).

Figure 1. Cumulative ammonia-nitrogen emission (g) and manure δ 15N (‰) from 15 days of dairy manure incubation. CT: UN:FN = 1:1; 2U1F: UN:FN = 2:1; CT + L: CT + Lignite; 2U1F + L: 2U1F + Lignite. CT used a manure with UN:FN = 1:1; 2U1F used a manure with UN:FN = 2:1; CT + L used a manure with UN:FN = 1:1 + 250 g lignite application; 2UIF + L used a manure with UN:FN = 2:1 + 250 g lignite application; UN:FN, urinary nitrogen: faecal nitrogen.
Cumulative ammonia-nitrogen emission, and nitrogen to phosphorus and potassium ratios
The cumulative NH3-N emission and manure N: P ratio from CT treatment had a relatively strong relationship (R 2 = 0.67, P ≤ 0.001) while it was moderate from the 2U1F treatment (R 2 = 0.54, P ≤ 0.001; Fig. 2). In the lignite group, the cumulative NH3-N emission and manure N: P had strong relationships (R 2 = 0.73 and 0.85 in CT + L and 2U1F + L treatments, P ≤ 0.001).

Figure 2. Cumulative ammonia-nitrogen emission (g) and nitrogen to phosphorus ratio (g/g) from 15 days of dairy manure incubation. CT: UN:FN = 1:1; 2U1F: UN:FN = 2:1; CT + L: CT + Lignite; 2U1F + L: 2U1F + Lignite. CT used a manure with UN:FN = 1:1; 2U1F used a manure with UN:FN = 2:1; CT + L used a manure with UN:FN = 1:1 + 250 g lignite application; 2UIF + L used a manure with UN:FN = 2:1 + 250 g lignite application; UN:FN, urinary nitrogen: faecal nitrogen.
The cumulative NH3-N emission and manure N: K ratio from the 2U1F treatment had a strong relationship (R 2 = 0.84, P ≤ 0.001) while it was moderate from the CT treatment (R 2 = 0.48, P ≤ 0.001; Fig. 3). In the lignite group, cumulative NH3-N emission and manure N: K ratio relationship was weak in CT + L treatment (R 2 = 0.64, P ≤ 0.001) and was relatively strong in 2U1F + L treatment (R 2 = 0.34, P ≤ 0.001).

Figure 3. Cumulative ammonia-nitrogen emission (g) and the ratio of nitrogen and potassium (g/g) from 15 days dairy manure incubation. CT: UN:FN = 1:1; 2U1F: UN:FN = 2:1; CT + L: CT + Lignite; 2U1F + L: 2U1F + Lignite. CT used a manure with UN:FN = 1:1; 2U1F used a manure with UN:FN = 2:1; CT + L used a manure with UN:FN = 1:1 + 250 g lignite application; 2UIF + L used a manure with UN:FN = 2:1 + 250 g lignite application; UN:FN, urinary nitrogen: faecal nitrogen.
Discussion
The experimental design drew inspiration from the NB study and previous research by Spanghero and Kowalski (Reference Spanghero and Kowalski1997), establishing UN and FN outputs as key metrics. In the non-lignite group, NH3-N emissions exhibited a decreasing trend over incubation days, driven by diminishing urinary urea concentration and hydrolysis.
The major NH3-N emission occurred in the last 10 days in lignite groups. This could be caused by lignite reaching the maximum capacity to suppress NH3-N emission. The increasing trend agreed with the results of Impraim et al. (Reference Impraim, Weatherley, Coates, Chen and Suter2020). Although Impraim et al. (Reference Impraim, Weatherley, Coates, Chen and Suter2020) found the same trend of the daily NH3-N emission and temperature, the weak relationships between temperature and daily NH3-N emission in four treatments (R 2 = 0.30) were observed in our experiment. This modest correlation may be attributed to the controlled environmental conditions within the laboratory setting, wherein temperature fluctuations were limited to a narrow range. It is plausible that a broader spectrum of temperature variations would yield a more pronounced relationship. Therefore, expanding the range of temperature fluctuations is anticipated to enhance the strength of the observed association. The manure δ 15N from the CT and 2U1F treatments had a strong relationship with incubation days, similar relationships were reported in studies by Hristov et al. (Reference Hristov, Zaman, Vander Pol, Ndegwa, Campbell and Silva2009) and Lee et al. (Reference Lee, Hristov, Cassidy and Heyler2011).
The manure N: P ratio from four treatments had a strong relationship with incubation days (R 2 = 0.84, 0.73, 0.81, and 0.84 for CT, 2U1F, CT + L, and 2U1F + L treatments, respectively). This strong relationship was also proved by Moreira and Satter (Reference Moreira and Satter2006), who stated that the N: P ratio decreased from 6.57 g/g in fresh manure to 2.33 g/g in manure incubated after 30 days. The weak relationship between manure N: K ratio and incubation days may be caused by one outliner data on day 15; however, the relationships were strong in the other three treatments. Similar to the manure N: P ratio, the manure N: K ratio decreased with incubation days in four treatments, which were also approved by Moreira and Satter (Reference Moreira and Satter2006) and Hristov et al. (Reference Hristov, Zaman, Vander Pol, Ndegwa, Campbell and Silva2009).
The strong relationship between cumulative NH3-N emission and manure δ 15N from the non-lignite group (combined CT and 2U1F treatments) were also reported by previous studies (Hristov et al., Reference Hristov, Zaman, Vander Pol, Ndegwa, Campbell and Silva2009; Lee et al., Reference Lee, Hristov, Cassidy and Heyler2011; Cheng et al., Reference Cheng, Sheahan, Gibbs, Rius, Kay, Meier, Edwards, Dewhurst and Roche2013; Ayadi et al., Reference Ayadi, Cortus, Clay and Hansen2015). Although Moreira and Satter (Reference Moreira and Satter2006) used the manure N: P and N: K ratios to estimate NH3-N emission, the moderate relationships were observed from 2U1F treatment in manure N: P ratio and from CT treatment in manure N: K ratio. The manure δ 15N could be the best biomarker used to estimate cumulative NH3-N emission under normal NH3-N concentrations.
Furthermore, the literature reviewed did not provide any references pertaining to the efficacy of N: P ratio, N: K ratio and δ 15N in mitigating NH3 emissions at concentrations below 0.15 g per 24 h- the upper threshold applied in lignite application modalities within our experimental framework. No relationship was found between cumulative NH3-N emission and manure δ 15N when combined CT + L and 2U1F + L treatments (Lignite group; R 2 = 0.02). This suggests that manure δ 15N may not serve as a preferable or accurate biomarker for estimating NH3-N emissions in scenarios involving lignite. However, strong relationships were identified between cumulative NH3-N emissions and manure N: P ratio under low NH3-N concentrations. Conversely, the relationship between cumulative NH3-N emissions and manure N: K ratio was weak in CT + L treatment but relatively strong in the 2U1F + L treatment. The manure N: P ratio emerged as a more accurate and effective biomarker for estimating NH3-N emitted under conditions of low NH3-N concentration. Therefore, further research is needed to validate the use of δ 15N to estimate NH3 emissions under laboratory and field conditions, accounting for variations in cattle manure properties and climatic conditions.
The findings from this experiment have significant implications for understanding NH3-N emissions from cattle manure, particularly in relation to lignite application. Understanding the underlying physical and biological processes leading to fractionation is crucial for accurate interpretation of isotopic data. Unravelling these mechanisms will enhance the precision of isotopic markers in assessing NH3-N emissions. The experiment introduces the manure N: P ratio as a robust biomarker for NH3-N emissions, outperforming other indicators under low NH3-N concentrations. This has practical implications for developing more accurate estimation models, guiding manure management decisions, and potentially providing a more nuanced understanding of emission dynamics.
Future research should focus on the following objectives: (1) refining isotopic markers and improving their accuracy in estimating NH3-N emissions requires a thorough understanding of the underlying physical and biological mechanisms of N fractionation; (2) it is necessary to validate the effectiveness of identified biomarkers (i.e., N: P ratio, N: K ratio, δ 15N), under diverse climatic conditions, cattle manure properties, and management practices. These field studies will enhance the practical applicability of these biomarkers; (3) the utility and reliability of isotopic markers, particularly δ 15N, should be examined under conditions of low NH3-N emissions, as observed with lignite application treatments. Understanding the limitations of these markers will inform their appropriate use in various scenarios; and (4) creating integrated models that combine various biomarkers and environmental variables is crucial for accurately predicting NH3-N emissions. This will contribute to the development of more comprehensive and precise strategies for managing and mitigating NH3-N emissions from cattle manure.
Conclusion
The experiment demonstrated a linear increase in manure δ 15N with rising NH3 emissions and a decrease in the N: P ratio under specific treatments, indicating these markers complementary roles. The δ 15N was effective at high NH3 concentrations, while the N: P ratio reliably estimated NH3 emissions. These findings highlight the potential of lignite application as a mitigation strategy and suggest further research to optimize its use in livestock industries.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0021859624000467
Acknowledgements
The study was supported by the Faculty of Science, the University of Melbourne. The authors express gratitude to various contributors from the University of Melbourne, including Dr Ravneet Kaur Jhajj (Dookie college lab manager) and Michael Hall (Trace Analysis for Chemical, Earth, and Environmental Science (TrACEES) platform) for their technical assistance. Special thanks to A/Prof Graham Brodie for his help in developing the study methodology.
Authors’ contributions
Song, Y.: Conceptualization, Methodology, Conducted data gathering, Investigation, Formal analysis, Writing – original draft, Writing – Editing. Bai, M.: Conceptualization, Methodology, Investigation, Reviewing and Editing. Chen, D.: Funding acquisition, Conceptualization, Methodology, Reviewing and Editing. Khanaki, H.: Conducted data gathering, Investigation, Reviewing, Editing and formatting. Cheng, L.: Funding acquisition, Conceptualization, Methodology, Investigation, Formal analysis, Reviewing and Editing.
Funding statement
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical standards
The experiment was approved by The Animal Ethics Committee from the Faculty of Science, the University of Melbourne, Australia (No. 1914778.1).
Data availability
Data will be made available on request.







