Long-chain n-3 polyunsaturated fatty acids (n-3 LCP) may influence heart rate variability (HRV), which might explain why very low intakes are associated with increased risk of sudden cardiac death( Reference Christensen 1 ). Lethal arrhythmias are associated with increased sympathetic and decreased parasympathetic autonomic activity which can be non-invasively assessed by various HRV parameters( Reference Malik 2 ). We report the effects of supplementation with n-3 LCP on sleep-time HRV in non-smoking men and women (aged 45–70 y) without medical history of cardiovascular disease.
The participants were drawn from the MARINA trial (ISRCTN66664610) which compared intakes of 0.45, 0.9 and 1.8 g/d of a mixture of EPA+DHA-rich triacylglycerols provided in 3 soft gel capsules/d with matched placebo capsules containing an oleic acid-rich triacylglycerol over a one-year period( Reference Sanders, Hall and Maniou 3 ). Participants were requested to restrict their intake of oily fish and to avoid taking any other dietary supplements. Actiheart monitors (CamNtech Ltd, Cambridge, UK) were fitted following a clinic visit and heart rate interbeat intervals were recorded over 24 h following a 1 month run-in on placebo and at 6 and 12 months following randomisation to treatment. Participants completed a sleep record during the measurement periods. Data artefacts due to signal detection problems were removed using Actiheart 4 (v. 4.0.91) and Kubios HRV software (v. 2.0, Biosignal Analysis and Medical Imaging Group, University of Kuopio, Finland). Time domain, frequency domain and non-linear HRV parameters were available for 231 out of 312 participants (male n 94, female n 137).
The following HRV parameters (frequency domain, time domain and non-linear) are reported: SDNN and triangular index (Ti), estimates of overall variability; high frequency (HF) power, a short-term component of HRV reflecting vagal modulation; and SDANN and very low frequency (VLF) power, indicating longer-phase variability. Results are shown in Table 1. SDANN, VLF and Ti increased during treatment. No dose-response was observed.
Table 1. Treatment effect versus placebo (average of change following each treatment divided by change on placebo at 6 and 12 months), based on estimated marginal means (adjusted for covariates: age, sex, ethnicity, BMI, and the value at baseline).*Placebo (n 52), ref. 1.000. The P value is derived from a linear trend test based on a weighted combination (0:1:2:4) Geometric means (95% CI) on placebo at baseline and follow-up were: SDNN baseline 95.0 (86.8, 103.9) ms, follow-up 87.7 (81.4, 94.6) ms, Ti baseline 21.6 (19.4, 24.1), follow-up 20.0 (18.5, 21.6), HF baseline 324 (260, 404) ms2, follow-up 347 (275, 439) ms2, SDANN baseline 76.3 (68.0, 85.6) ms, follow-up 67.2 (61.0, 74.0) ms, VLF baseline 5363 (4493, 6403) ms2, follow-up 4444 (3844, 5138) ms2
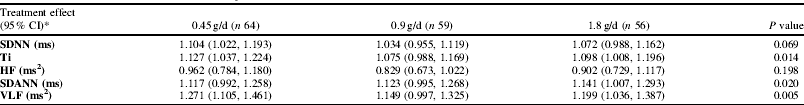
This study indicates increases in the longer-phase components of night time HRV in the n-3 LCP supplemented groups compared to placebo. The main effect was in the comparison with the change on placebo rather than between dose levels. This would support the view that there may be a very low threshold effect for n-3 LCP intake in relation to HRV.
Funding for this work was provided by the Food Standards Agency and the Department of Health, England (Project code N02041).



