Schizophrenia, which is characterised by disturbances in cognition, affect, perception and behaviours, affects approximately 1% of the population;Reference Saha, Chant, Welham and McGrath1 however, schizophrenia can be difficult to treat, even with optimal therapy. Roughly one-third of people with schizophrenia have treatment-resistant schizophrenia (TRS),Reference Siskind, Orr, Sinha, Yu, Brijball and Warren2 defined as ongoing symptoms and functional impairment despite two adequate and adherent trials of different antipsychotics.Reference Howes, McCutcheon, Agid, de Bartolomeis, van Beveren and Birnbaum3
Although antipsychotic medications and supportive therapies reduce symptom burden and relapse rate, many individuals with schizophrenia continue to have ongoing symptoms and concomitant cognitive and social disabilities, poor physical health and curtailed life expectancy.Reference Lieberman, Stroup, McEvoy, Swartz, Rosenheck and Perkins4 Currently, the most effective antipsychotic for TRS is clozapine, which leads to reductions in positive symptoms and hospital admissions.Reference Land, Siskind, McArdle, Kisely, Winckel and Hollingworth5,Reference Siskind, McCartney, Goldschlager and Kisely6 Even so, only 40% of people with TRS trialled on clozapine meet clinical response criteria.Reference Siskind, Siskind and Kisely7 For people with clozapine-resistant schizophrenia, there are few agents available to augment treatment, and these have limited effectiveness.Reference Siskind, Lee, Ravindran, Zhang, Ma and Motamarri8
The endocannabinoid system has a strong neuromodulatory role, with effects on inflammation, cell proliferation and apoptosis, pain modulation, memory and learning, and fear, among other functions.Reference Lu and Mackie9 Endocannabinoids bind to presynaptic receptors, including CB1 and CB2, to potentiate retrograde signals that regulate the release of other neurotransmitters, such as dopaminergic, serotonergic, adrenergic, cholinergic and γ-aminobutyric acid (GABA).Reference Fasinu, Phillips, ElSohly and Walker10,Reference Pacher, Bátkai and Kunos11 Cannabidiol (CBD) is the non-intoxicating component of the cannabis plant. Compared with delta-9-tetrahydrocannabinol (THC), CBD has a more anxiolytic profile, sparing disturbances in perception and thought processing.Reference Pisanti, Malfitano, Ciaglia, Lamberti, Ranieri and Cuomo12 CBD has strong potential for neuropsychiatric therapeutic use, as it acts on the central nervous system via neuroreceptors including CB1, the serotonin 5-HT1A receptor and the transient receptor potential vanilloid type 1 receptor.Reference Blessing, Steenkamp, Manzanares and Marmar13 Recent studies have explored the potential of CBD to target the endocannabinoid system to improve mood and anxiety, epilepsy, chronic pain, movement disorders (such as Parkinson's disease), nausea and inflammation associated with metabolic syndrome, malignancy, atherosclerosis, etc.Reference Pacher, Bátkai and Kunos11,Reference Blessing, Steenkamp, Manzanares and Marmar13 Following animal and in vivo studies, it has been suggested that CBD is non-toxic, with minimal (usually mild) side-effects.Reference Wang, Collet, Shapiro and Ware14 CBD has shown promise as a treatment for mental disorders, including anxiety, insomnia and post-traumatic stress disorder.Reference Sarris, Sinclair, Karamacoska, Davidson and Firth15
There have been three randomised placebo-controlled trials, albeit not among people with clozapine-resistant schizophrenia.Reference Boggs, Surti, Gupta, Gupta, Niciu and Pittman16–Reference Leweke, Piomelli, Pahlisch, Muhl, Gerth and Hoyer18 A 6-week double-blind parallel-group trial of 88 participants with partially responsive schizophrenia, not trialled on clozapine, compared adjunctive CBD 1000 mg/day with placebo.Reference McGuire, Robson, Cubala, Vasile, Morrison and Barron17 This study found significantly greater improvements in positive psychotic symptoms in the CBD group. However, it failed to reach significance with respect to the difference in the proportions of participants in the two groups showing ≥20% improvement in PANSS (Positive and Negative Syndrome Scale) total score. A second 4-week blind randomised controlled trial (RCT) among 42 participants receiving 600–800 mg CBD daily versus the standard antipsychotic medication, amisulpride, showed improvements in psychotic symptoms with both treatments and fewer adverse drug reactions in the CBD arm.Reference Leweke, Piomelli, Pahlisch, Muhl, Gerth and Hoyer18 However, the third study, a double-blind 6-week randomised controlled trial in 36 treatment-responsive patients with schizophrenia did not find a difference in psychotic symptoms between groups receiving adjunctive CBD 600 mg/day and placebo.Reference Boggs, Surti, Gupta, Gupta, Niciu and Pittman16 Other non-randomised or single-dose CBD studies have shown equivocal results.Reference O'Neill, Wilson, Blest-Hopley, Annibale, Colizzi and Brammer19
CBD has been found to be well tolerated at different doses across several clinical trials. A trial by Taylor et al in healthy controls showed good tolerance of single CBD doses of 1500, 3000, 4500 and 6000 mg.Reference Taylor, Gidal, Blakey, Tayo and Morrison20 In patients receiving multiple doses (750 and 1500 mg twice daily) for 6 days, CBD was also well tolerated, with no participants leaving the trial owing to adverse events.Reference Taylor, Gidal, Blakey, Tayo and Morrison20 McGuire et al trialled doses of 1000 mg daily of CBD in patients with schizophrenia in addition to their usual antipsychotic medications for 6 weeks.Reference McGuire, Robson, Cubala, Vasile, Morrison and Barron17 Positive psychotic symptoms were lower in the treatment group, with similar adverse events to those of the placebo group, showing good tolerance for CBD.Reference McGuire, Robson, Cubala, Vasile, Morrison and Barron17
To date, there have been no trials of CBD among people with clozapine-resistant schizophrenia. This trial has the potential to identify CBD – if found to be effective – as a novel and safe adjunctive treatment for individuals with clozapine-resistant schizophrenia.
Study objectives
The aim of this study is to examine the clinical efficacy of an add-on CBD treatment in patients with clozapine-resistant schizophrenia. The primary objective is to determine the impact of 12-week treatment with CBD, compared with placebo, on scores on the positive subscale of PANSS in patients with clozapine-resistant schizophrenia.
Hypothesis
We hypothesise that participants allocated to the intervention group (1000 mg daily CBD treatment) will demonstrate a reduction in PANSS positive scores at the end of week 12 compared with participants receiving placebo.
Method
Design and setting
The study is a randomised, placebo-controlled, double-blind parallel-group adaptive trial to examine the clinical efficacy and safety of add-on CBD treatment in patients with clozapine-resistant schizophrenia. It will recruit 88 individuals with clozapine-resistant schizophrenia, who will be randomised at the point of providing written informed consent. The study design was discussed with our local lived experience reference group.
Participants will be given 1000 mg daily of CBD or placebo, in addition to routine care. A battery of validated clinical and physical health measures will be conducted throughout the trial period. Adverse events will be recorded fortnightly (i.e. weeks 0, 2, 4, 6, 8, 10 and 12), and trial medication will be distributed to participants fortnightly (i.e. weeks 0, 2, 4, 6, 8 and 10).
The authors assert that this study complies with the ethical standards of the relevant national and institutional committees on human experimentation and with the World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects.Reference World Medical Association21 All procedures involving human participants were approved by the Metro South Human Research Ethics Committee (reference number: HREC/2022/QMS/83530). The study sponsor is the University of Queensland, which provides overarching governance. We have separate site governance approvals for each recruiting site. The trial is registered on the Australia New Zealand Clinical Trials Registry (registration number: ACTRN12622001112752). The study will follow the CONSORT guidelines for parallel-group randomised trials.Reference Moher, Hopewell, Schulz, Montori, Gøtzsche and Devereaux22
Participants
Eighty-eight eligible participants with TRS will be recruited through mental health services at the Gold Coast, Metro North, Metro South, and West Moreton Hospital and Health Services and in south-east Queensland, Australia. Participant eligibility is detailed in Box 1. Participants must meet all the inclusion criteria and none of the exclusion criteria. Capacity to consent will be determined by the treating clinician in collaboration with clinical trial staff. Only participants with this capacity will be recruited following their provision of consent.
Box 1 Participant eligibility

Allocation concealment and randomisation
Participants will be randomised using a chronological process and only after written consent has been provided and baseline assessments have been completed. Participants will be randomised to either the intervention or control group in a 1:1 ratio using a computer-generated randomisation table with block randomisation. They will be allocated a unique identification number, which will be linked to a specific number based on the site they have been recruited from. If a participant withdraws from the study, their identification number will not be re-used, nor will they be allowed to re-enter the study.
Participants, clinical trial staff, investigators and treating clinicians will be blinded to the intervention. An independent biostatistician will generate the randomisation list, which will be provided to the manufacturer and a study site pharmacist. The pharmacist will hold the closed randomisation list and will be the only one who is able to unblind participants. In the case of an emergency, where it is critical that medical staff know whether a participant is on CBD or placebo, participants will be provided with contact information for unblinding (i.e. a 24-h number).
Pharmacological treatment
Participants will receive five gel capsules per day containing either 200 mg CBD (99% purity; <0.1 mg THC per capsule) suspended in medium-chain triglyceride oil or matched placebo (medium-chain triglyceride oil only). Capsules were manufactured by Linnea SA, Lavertezzo, Switzerland, a Therapeutic Goods Administration licensed facility, in accordance with current Good Manufacturing Practice and distributed by BOD Science. Dispensation, by a clinical trial staff member, will occur on a fortnightly basis, with six dispensations total per participant.
Outcomes
Primary
The primary outcome was the impact of 1000 mg daily CBD treatment over 12 weeks compared with placebo on PANSS positive score in participants with clozapine-resistant schizophrenia.
Secondary
Secondary outcomes included the impact of 1000 mg daily CBD treatment over 12 weeks compared with placebo on PANSS total scores and other PANSS subscales; depression; anxiety; sleep; quality of life; alcohol consumption; changes in weight and metabolic syndrome components including waist circumference, haemoglobin A1C (HbA1c), high-density lipoprotein (HDL), low-density lipoprotein (LDL), body mass index (BMI), triglycerides, blood pressure, hip-to-waist ratio, electrolyte and liver function tests (ELFTs), non-alcoholic fatty liver disease (NAFLD) scores, fibrosis-4 scores (FIB-4), heart rate, diet and appetite, and physical activity; endpoint clozapine and norclozapine levels compared with baseline; and neurocognitive measures. Additional aims were to determine the safety and tolerability over 12 weeks of 1000 mg daily CBD in patients with clozapine-resistant schizophrenia compared with placebo as measured by the number of participants reported to have dropped out, number of adverse events reported, and scores from a structured qualitative interview with participants about their experience with the study investigational product using the Systematic Assessment for Treatment Emergent Events – Systematic Inquiry (SAFTEE-SI).Reference Rabkin, Markowitz, Ocepek-Welikson and Wager24
Tertiary
Tertiary outcomes were changes between baseline and the 12-week endpoint in CBD and CBD metabolites (7-COOH-CBD, 6-OH-CBD, 7-OH-CBD), medication adherence, and measurements of THC and its metabolites (11-COOH-THC, 11-OH-THC) to check for possible illicit cannabis use.
Measurements
Table 1 illustrates the trial schedule of visits and assessments. A range of validated clinical and physical health measures will be administered at different points throughout the trial period. Clinical measures will be administered by clinical trial staff, whom the clinical trial research manager will supervise. These staff are experienced mental health clinicians trained to administer these measures. Depending on participants' preferences, clinical measures will be administered in the clinic or at the participant's residence.
Table 1 Schedule of trial visits and assessments
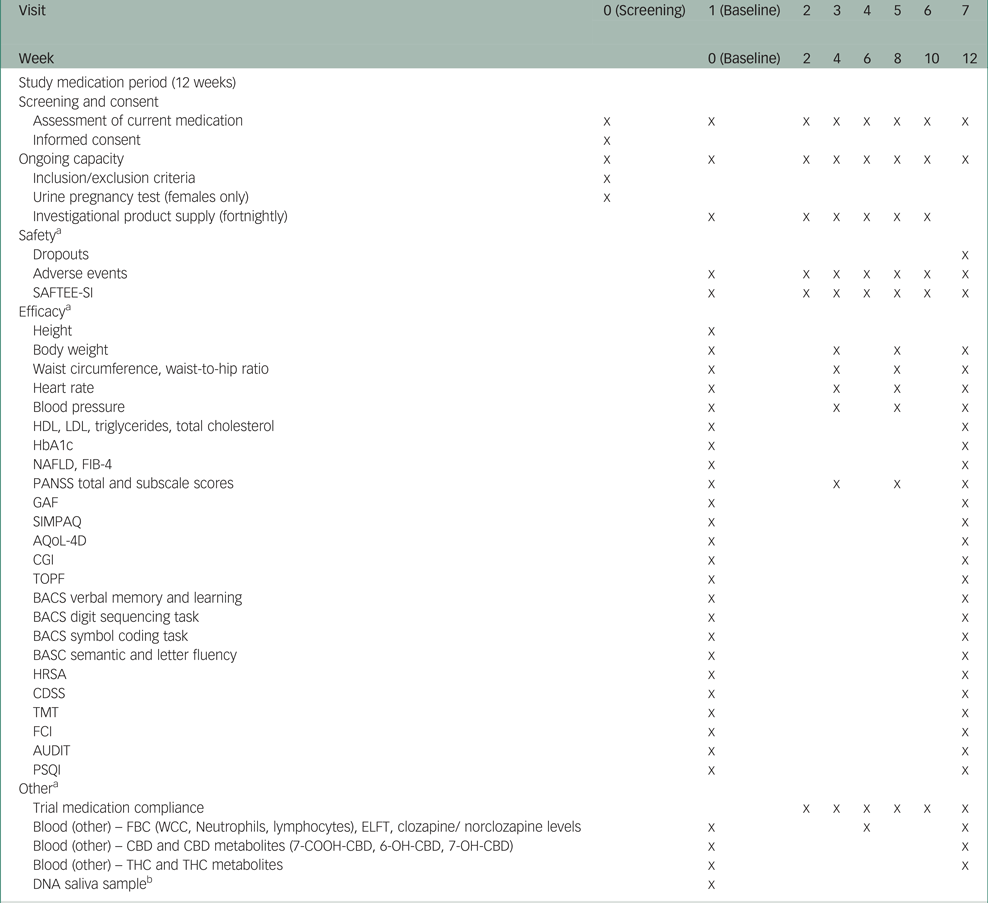
SAFTEE-SI, Systematic Assessment for Treatment Emergent Events – Systematic Inquiry; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HbA1c, glycated haemoglobin; ELFT, electrolyte and liver function tests; NAFLD, non-alcoholic fatty liver disease; FIB-4, fibrosis-4; PANSS, Positive and Negative Syndrome Scale; GAF, Global Assessment of Function; SIMPAQ, Simple Physical Activity Questionnaire; AQoL, Assessment of Quality of Life; CGI, Clinical Global Impression; TOPF, Test of Premorbid Function; BACS, Brief Assessment of Cognition in Schizophrenia; HRSA, Hamilton Rating Scale for Anxiety; CDSS, Calgary Depression Scale for Schizophrenia; TMT, trail making test; FCI, Food Craving Inventory; AUDIT, Alcohol Use Disorders Identification Test; PSQI, Pittsburgh Sleep Quality Index; FBC, full blood count; WCC, white cell count; CBD, cannabidiol; THC, tetrahydrocannabinol.
a. Assessment schedule may vary by plus or minus 5 days for operational convenience.
b. Providing a saliva sample is optional for participants and will be used to explore the presence of known correlates of clozapine and obesity, including variants in genes such as LEP and HTR2C.
Efficacy measures
The PANSS positive score will be the primary outcome.Reference Kay, Fiszbein and Opler26 Secondary outcome measures will include the following:
• PANSS total and other subscale scores (negative scale and general psychopathology scale);Reference Kay, Fiszbein and Opler26
• Global Assessment of Function: a numeric scale from 1 to 100 used by clinicians to subjectively rate participants' social, occupational and psychological functioning;Reference Hall27
• Simple Physical Activity Questionnaire: a five-item instrument designed to measure physical activity and sedentary behaviour in various populations, including clinical samples with high levels of sedentary behaviour;Reference Rosenbaum, Morell, Abdel-Baki, Ahmadpanah, Anilkumar and Baie28
• Assessment of Quality of Life 4 Dimensions: a 12-item instrument that captures four dimensions of quality of life, comprising independent living, mental health, relationships and senses;Reference Richardson, Lezzi, Khan, Sinha, Mihalopoulos and Herrman29
• Clinical Global Impression: a measure of symptom severity, treatment response and treatment efficacy in treatment studies of people with schizophrenia;Reference Busner and Targum30
• Test of Premorbid Functioning: a measure of pre-injury IQ and memory ability;Reference Holdnack and Drozdick31
• Brief Assessment of Cognition in Schizophrenia (BACS) Verbal Memory and Learning: a measure of episodic verbal learning memory;Reference Keefe, Harvey, Goldberg, Gold, Walker and Kennel32
• BACS digit sequencing task: a measure of working memory;Reference Keefe, Harvey, Goldberg, Gold, Walker and Kennel32
• BACS symbol coding task: a measure of non-verbal functions such as attention, flexibility, speed of processing and abstract reasoning;Reference Keefe, Harvey, Goldberg, Gold, Walker and Kennel32
• BACS semantic and letter fluency: a verbal fluency test that measures spontaneous production of words that belong to the same category or begin with the same letter;Reference Keefe, Harvey, Goldberg, Gold, Walker and Kennel32
• Hamilton Rating Scale for Anxiety: a measure of anxiety symptom severity;Reference Hamilton33
• Calgary Depression Scale for Schizophrenia: a nine-item measure of depression in schizophrenia, independent of psychosis symptoms;Reference Addington, Addington and Schissel34
• Trail Making Test: a neuropsychological test of visual attention and task switching that can provide information about visual search speed, scanning and speed of processing, mental flexibility, and executive functioning;Reference Gaudino, Geisler and Squires35
• Food Craving Inventory: a 28-item self-reported measure of general and specific food cravings;Reference White, Whisenhunt, Williamson, Greenway and Netemeyer36
• Alcohol Use Disorders Identification Test: a ten-item screening tool to assess alcohol consumption, behaviours, and problems associated with alcohol use;Reference Saunders, Aasland, Babor, De La Fuente and Grant37
• Pittsburgh Sleep Quality Index: a 19-item self-reported measure of overall sleep quality assessing seven domains comprising subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication and daytime dysfunction;Reference Buysse, Reynolds, Monk, Berman and Kupfer38
• physical health measures including, BMI, waist circumference, waist-to-hip ratio, heart rate, blood pressure, HDL, LDL, triglycerides, total cholesterol, HbA1c, ELFTs, NAFLD and FIB-4.
Other measures
In addition to the abovementioned measures, we will assess adherence to the trial investigational product. This will be based on self-report and capsule count at each visit. We will perform additional blood tests including a full blood count and measure clozapine/ norclozapine levels, as well as levels of CBD, THC and their metabolites.
Participants will also have the option of providing a saliva sample at baseline. This will be used to explore the presence of known genetic correlates of clozapine and obesity, including variants in genes such as LEP and HTR2C.Reference Vasudev, Choi, Norman, Kim and Schwarz25 The DNA samples collected in this study will be used to validate associations between DNA single-nucleotide polymorphisms and treatment-resistant clozapine patient populations.
Our research team has used this suite of measures in other clinical trialsReference Siskind, Russell, Suetani, Flaws, Kisely and Moudgil39–Reference Siskind, Russell, Gamble, Winckel, Mayfield and Hollingworth41 and found them to be acceptable to study participants with schizophrenia, with low rates of dropout due to burden of clinical measures.
Statistical analysis
Sample size
A power analysis was conducted based on the mean difference in positive PANSS score between treatment arms at the primary analysis endpoint (12 weeks post-baseline assessment). A mean difference in positive PANSS score of 1.30 at 8 weeksReference McGuire, Robson, Cubala, Vasile, Morrison and Barron17 (extrapolated to a conservative difference of −1.8 at 12 weeks, assuming a logarithmic trend over a linear trend), standard deviation difference of 3.739 (Cohen's d effect size of 0.48), correlation between assessment timepoints of 0.6, α = 0.025 (one-sided), and 1 − β = 0.8, with repeated measures (four assessment timepoints) and analysis of variance (ANOVA) as the planned analysis with pairwise contrast, yielded a requirement of 70 participants. Allowing for 20% dropout and rounding up to the nearest multiple of treatment arms (n = 2) suggests that a minimum sample of 88 participants is required overall.
Data analysis
The effect of CBD relative to placebo on the outcome of 12-week positive PANSS score will be analysed using mixed model repeated measures with fixed effects for treatment arm, time, study site, and treatment arm × time interaction. A random intercept will be included to account for variation in scores between participants. An unstructured variance–covariance structure will be assumed, and where there are convergence issues, a compound symmetry covariance structure will be used instead. Consistent with a treatment policy approach (ICH E9 R1), all participant data will be analysed regardless of missed assessment data, withdrawal from the treatment arm or use of rescue medication (i.e. intention to treat). Suitable contrasts will be used to evaluate the difference in mean total PANSS scores at the primary endpoint (week 12). Missing data will be imputed using multiple imputation by chained equations (MICE) under an assumption of missing at random. A sensitivity analysis will be conducted using a tipping point analysis and the delta adjustment method (e.g. MICE ± 1 PANSS positive score point increments) to evaluate the robustness of the model to the underlying assumptions of missingness. A further sensitivity analysis will be conducted using an alternative analysis of covariance model plus MICE. All other secondary and/or exploratory outcomes will be analysed using the same approach. The number needed to treat will be calculated based on the proportion of participants in each arm who achieve >20% reduction in total PANSS score. Use of co-medications, clozapine/norclozapine levels, and presence of THC and THC metabolites will be considered in the analysis.
Interim analysis
Three interim analyses will be performed at 25%, 50% and 75% recruitment, corresponding to n = 22, 44 and 66 participants having completed their 12-week assessments. Each interim analysis will provide an ospportunity to evaluate whether the trial can be concluded at an earlier stage, rather than progressing until the target sample has been fully recruited. The interim analysis will evaluate the level of efficacy achieved, conditional on a linear trajectory of change in PANSS positive scores and an end-of-trial Cohen's d effect size of 0.48. The upper (early efficacy) and lower (early futility) bounds and their corresponding mean difference at interim analysis are summarised in Table 2. Conditional power at each interim analysis will be evaluated using bootstrap resampling (n = 10 000). Dependent on conditional power at interim analyses, we will consider randomisation reallocation to address the level of imbalance to achieve the desired effect size. The interim analysis will also provide an opportunity to reallocate the proportion of participants allocated to placebo and CBD if the study is found to be underpowered. Results of each interim analysis will be non-binding, with results of interim analyses provided to the Data Safety Monitoring Board (DSMB) for further evaluation.
Table 2 Summary interim analysis upper and lower bounds

βc = probability of type II error at differing time points; δ, mean difference achieved at interim analysis.
Participant safety
To assess the preliminary safety and tolerability of 1000 mg daily CBD for 12 weeks in people with clozapine-resistant schizophrenia (secondary objective), we will assess:
(a) the dropout rate between intervention and control groups;
(b) the numbers of adverse events reported in the intervention and control groups;
(c) liver function test results at baseline and weeks 6 and 12;
(d) scores from a structured qualitative interview with participants about their experience with the trial investigational product using the SAFTEE-SI.
All participants will be currently active cases across Queensland Hospital and Health Services. The research team will communicate with clinicians to guarantee that all participants have undergone a regular biannual physical health assessment as part of their standard care. The investigator and clinical trial staff will oversee any adverse events reported during the study. Any adverse events reported between participant consent and final follow-up will be recorded using designated case report forms.
Patient withdrawal
All participants have the right to withdraw their consent at any time without prejudice or effect on their ongoing care. This will be clearly discussed during the consent process. If a participant decides to withdraw consent, the trial team will complete a revocation of informed consent form.
Reimbursement
Participants will be reimbursed for out-of-pocket expenses, inconvenience and time involved in the trial through the provision of prepaid gift cards. We will provide an AU$20 gift card at the baseline visit and at each fortnightly visit thereafter (total reimbursement AU$140). If the study is terminated by the research team before completion, or a participant withdraws or is withdrawn from the study before completion, a pro rata payment will be made at the discretion of the investigator.
Transparency declaration
D.S. (lead author and manuscript guarantor) affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned and registered have been explained.
Discussion
Schizophrenia remains one of the most complex and poorly understood psychiatric disorders. This trial seeks to generate new knowledge to support people with schizophrenia by determining the efficacy of add-on 1000 mg daily CBD for treatment of clozapine-resistant schizophrenia. Existing evidence suggests that CBD is well tolerated in this population with minimal side-effects.Reference Iffland and Grotenhermen42,Reference Deiana43
This trial has some limitations. First, there is no assurance that the study's findings will be generalisable to all individuals with clozapine-resistant schizophrenia. Although the proposed study is sufficiently powered to detect statistically significant differences between groups, the sample size (n = 88) is still small. Moreover, as the study sample is required to fulfil eligibility criteria such as abstinence from substances including amphetamine and cannabis, it may not be entirely representative of the real-world population.
This protocol provides a detailed account of the objectives, design, procedures and analytical approach of this trial. We anticipate that the results of the trial will show significant improvements in participants’ PANSS positive scores and will therefore inform clinical guidance on the maintenance treatment of clozapine-resistant schizophrenia. Moreover, we believe that this trial has the potential to minimise symptom severity and optimise quality of life for individuals with clozapine-resistant schizophrenia.
Data availability
The data that support the findings of this study will be available from the corresponding author upon reasonable request.
Acknowledgements
We thank all the patients and their family members, carers and friends for their participation in this study.
Author contributions
D.S., N.W., A.B. and I.M. conceived the original study idea and formulated the research questions. D.S., N.W., A.B. and I.M. led the study design with input from all authors. D.S., A.B. and C.B. worked on the initial draft of this manuscript with input from all authors.
Funding
This work was supported by the Princess Alexandra Hospital Foundation (D.S. (RSS_2021_020)) and the Lambert Initiative for Cannabinoid Therapeutics at the University of Sydney. The manufacturer of the CBD is not involved in the design of the study. The CBD and placebo were supplied for this trial by the Lambert Initiative for Cannabinoid Therapeutics at the University of Sydney.
Declaration of interest
S.K. is a member of the BJPsych Open Editorial Board. He did not take part in the journal's review or decision-making process regarding this submission.






eLetters
No eLetters have been published for this article.