Anorexia nervosa usually develops in early adolescence but over 50% of cases have an illness that persists for over 7 years and extends into adulthood. Reference Stoving, Andries, Brixen, Bilenberg and Horder1 The treatment response differs according to the stage of illness. Reference Treasure, Stein and Maguire2 For example, family-based therapy is more effective in the early stage of illness. Reference Fisher, Hetrick and Rushford3 The absolute rate of full recovery is less than one-third overtime. Reference Eddy and Gray4,Reference Le Grange, Lock, Accurso, Agras, Darcy and Forsberg5
Individuals with high levels of medical risk are managed by specialised in-patient care. The mortality, morbidity and service use and cost for in-patient care for anorexia nervosa are amongst the highest of all psychiatric disorders. Reference Chesney, Goodwin and Fazel6–Reference Green and Griffiths8 Protracted support from both the state and/or family is needed for people with the severe enduring form of illness. Reference Hjern, Lindberg and Lindbald9 Family members experience high levels of burden and distress Reference Anastasiadou, Medina-Pradas, Sepulveda and Treasure10,Reference Zabala, Macdonald and Treasure11 and request information and help with their caregiving role. Reference De Zwaan, Zipfel, Herzog, Herpertz-Dahlmann, Konrad and Hebebrand12–Reference Haigh and Treasure14 We have developed a skills training intervention (Experienced Caregivers Helping Others (ECHO)) to support the caregiving role at home based on caregivers’ needs and our cognitive interpersonal model of anorexia nervosa. Reference Schmidt and Treasure15,Reference Treasure and Schmidt16
The aim of this study was to evaluate the impact of the addition of the ECHO intervention on patient and caregivers well-being following in-patient care. Families were randomised to receive ECHO plus treatment as usual (TAU) or TAU alone at the time patients were admitted to hospital. Caregiver and patient well-being was followed in the year following discharge. The trial investigated the following hypotheses regarding patient (P) and caregiver (C) outcomes. The primary patient hypothesis was that at 12-month follow-up, patients with caregivers allocated to ECHO would have a reduced rate of relapse compared to those allocated to TAU. The secondary hypothesis for patients was that patients with caregivers allocated to ECHO would sustain a higher body mass index (BMI) and quality of life and lower eating psychopathology (EDE-Q) and distress (Depression, Anxiety and Stress Scale-21 (DASS-21)) post-randomisation compared to those allocated to TAU. The primary hypothesis for caregivers was that caregivers allocated to ECHO would have less distress (depression, anxiety and stress DASS) in the year after discharge compared to those allocated to TAU. The secondary hypothesis for caregivers was that those who receive ECHO will report lower expressed emotion, accommodation and enabling, caregiving burden, time spent caregiving and improved quality of life post-randomisation compared to those allocated to TAU.
Method
This was a pragmatic, two-arm, multicentre parallel group randomised controlled trial (RCT). A detailed account of the trial protocol, including the description of the ECHO caregiver intervention, has been published. Reference Goddard, Raenker, Macdonald, Todd, Beecham and Naumann17 Consenting caregivers of patients who met eligibility criteria were randomly allocated to receive either ECHO (in addition to treatment as usual (TAU)) or TAU only. Guidance for the ECHO intervention was delivered by ‘experienced’ coaches (people with lived experience of eating disorders, n=15) and also (in order to have a sufficient numbers) post-graduate-level psychologists (without clinical training, n=5) who were specifically trained and supervised. Patients and their caregivers were recruited from 15 hospital units providing National Health Service (NHS) in-patient/day-patient care for people with eating disorders. Outcome measures were collected at baseline, discharge, and 6 and 12 months following discharge.
Ethics approval was granted by the Royal Free Hospital Ethics Committee (08/H0720/41) with site-specific ethics and governance approval for all participating sites. The study was adopted by the Mental Health Research Network (MHRN). Following informed consent (from caregiver and patient) and completion of baseline assessment (on admission to the treatment facility), caregivers of a patient were randomly allocated to ECHO or TAU using an online system. Randomisation was carried out independently by the King's Clinical Trials Unit at King's College London and employed minimisation with stratification factors, study site (n=15) and disease severity (binary with the ‘severe’ category defined as one or both of body mass index (BMI) <15, presence of compensatory vomiting). The statisticians were masked to treatment allocation until adherence was measured.
Setting, recruitment and participants
Fourteen of the sites were eating disorder specialist in-patient wards in the UK (13 adults, 1 adolescent); one was a general (adolescent) psychiatric ward with eating disorder specialist staff. Four sites recruited day-patients as well as in-patients into the study. One of the specialist sites made local referrals to specialist eating disorder in-patient wards in the UK. Clinical Studies Officers (CSOs) from the MHRN supported recruitment of patients. Inclusion criteria: patients meeting the criteria of DSM-IV anorexia nervosa aged 12 years or above, able to speak and understand English. Exclusion criteria: no identified caregiver, patient/caregivers taking part in another treatment study or discharged from their in-patient stay before baseline assessment completed. In addition, participants with a severe comorbidity at time of admission (e.g. severe intellectual disability, physical illness, and psychosis) were not included in the study.
Interventions
Correspondence with caregivers on the randomisation outcome was by post. The ECHO group had a letter explaining that they would be contacted by their telephone coach and supporting documentation was sent (action/goal sheets, staging based on the transtheoretical model of change, Reference Prochaska and DiClemente18 frequently asked questions and troubleshooting for technical support). Those allocated to the TAU arm were given contact details for Beat, the leading UK eating disorder charity, and were offered access to the intervention on completion.
ECHO
Participants allocated to ECHO received this intervention as an addition to TAU. The materials were sent and the coaching begun immediately after randomisation. ECHO uses a skills training approach and consists of a book Reference Treasure, Smith and Crane19 and five DVDs (three theoretical, two practical). The DVDs complement the information presented in the book with role plays and practical examples in using a motivational interviewing style of communication and strategies to reduce expressed emotion and accommodation. The information on the DVDs is presented visually with audio voiceover. A more detailed description of DVD content and the coaching input is described elsewhere. Reference Goddard, Raenker, Macdonald, Todd, Beecham and Naumann17,Reference Sepulveda, Lopez, Macdonald and Treasure20 (A professionally produced version is available through the charity Succeed – www.succeedfoundation.org.)
In addition to the book and DVDs, the intervention package included five telephone coaching sessions per caregiver (up to 10 per family, e.g. mother and father). Single caregivers could receive up to 10 coaching calls. Participants were contacted by the coach within 2 weeks of receiving the material by post. Calls (of up to 40 min) were made by appointment approximately two weekly. To be classified as completing the intervention a minimum of four calls (per family) were delivered or 75% of the book read. Information on coaches, their training and measurement of quality assurance is described in the published protocol. Reference Goddard, Raenker, Macdonald, Todd, Beecham and Naumann17 The majority of coaches (64%) attained the recommended skill level for motivational interviewing. Reference Macdonald, Hibbs, Rhind, Harrison, Goddard and Raenker21
Treatment as usual
In-patient or day-patient treatment
The National Institute for Health and Care Excellence (NICE) guidelines have several Grade C recommendations about in-patient care. 22 The Royal College of Psychiatrists has developed accreditation standards which detail how caregivers should be involved in in-patient treatment. Reference Cresswell, Beavon and Robinson23 For this study, day-patients were defined as patients who required non-residential intensive specialist treatment (≥4 days a week).
Aftercare
The NICE guidelines specify that aftercare (focusing on eating behaviour) is provided for a year after discharge. This typically includes monitoring of physical risks, dietetic assessment and advice and some form of individual out-patient therapy. Cognitive–behavioural therapy, interpersonal psychotherapy or focal psychodynamic therapy are most commonly offered (see Results section for more detail).
Assessment measures
All participants (patients and caregivers) completed self-report assessments by post/telephone on admission to treatment unit, discharge from hospital and thereafter at 6 -and 12-month time point's post-discharge. For the year following discharge, patients additionally completed a short monthly assessment on core eating symptoms by telephone, email or post. Patient and caregiver primary and secondary outcomes are listed below. All measures have been validated in eating disorder populations and have good psychometric properties. See published protocol for more details. Reference Goddard, Raenker, Macdonald, Todd, Beecham and Naumann17
Patients’ assessment measures
Patients’ baseline assessment includes the following:
-
Sociodemographic features (a checklist used in pilot studies).
Patients’ clinical outcome measures include the following:
-
BMI (from clinical measures of height and weight).
-
Eating Disorder Examination Questionnaire (EDE-Q). Reference Luce and Crowther24 A self-report measure of eating disorder symptoms with good reliability and validity.
-
Depression, Anxiety and Stress Scale (DASS-21). Reference Lovibond and Lovibond25 A 21-item self-report measure validated in both clinical and non-clinical samples with good internal reliability.
-
World Health Organization – Quality of Life Questionnaire (WHO-Quol). 26 A self-report measure with good validity.
-
The Client Service Receipt Inventory (CSRI); Reference Beecham, Knapp and Thornicroft27 structured interview measuring use of specialist and generic health services.
Caregivers’ assessment measures include the following:
-
Burden: Eating Disorder Symptom Impact Scale (EDSIS): Reference Sepulveda, Whitney, Hankins and Treasure28 A 24-item self-report measure.
-
DASS. As described above.
-
Accommodation and Enabling Scale for Eating Disorders. Reference Sepulveda, Kyriacou and Treasure29 A 33-item self-report measure.
-
Family Questionnaire (FQ): this is a 20-item self-report measure assessing expressed emotion in caregivers using a 4-point Likert scale. Reference Wiedemann, Rayki, Feinstein and Hahlweg30
-
WHO-Quol. 26
-
The CSRI; Reference Beecham, Knapp and Thornicroft27 As above but caregiver version measuring time spent caregiving.
Statistical analysis
All statistical analyses were based on the intention-to-treat principle; participants were analysed in the treatment arm to which they were randomised. The primary patient clinical outcome was time from discharge to relapse. A linear change was assumed between monthly measurements of BMI in order to interpolate the day on which two points were estimated to be lost. This variable was right censored since relapse might not have occurred by the end of the study period (1 year) or follow-up data for BMI could be missing. If five consecutive BMI measurements were missed, time to relapse was considered censored. The primary caregiver clinical outcome was distress at 12 months after discharge. To account for two primary outcomes, group differences on these outcomes were tested at a significance level of 2.5%. Secondary caregiver and patient outcomes were continuous measures at discharge, 6-month or 1-year follow-up.
Time to relapse was defined as readmission to hospital due to their eating disorder or a drop of two points from discharge in BMI measured on a monthly basis (whichever came first) and analysed using Cox regression. Explanatory variables in this model were the variable of interest (treatment arm) and randomisation stratifiers (site and illness severity categories). The effect of treatment was estimated by the hazard ratio of relapse comparing ECHO with TAU.
The continuous secondary patient outcomes were analysed using linear mixed models. The dependent variable was the outcome at the respective time point (e.g. BMI at 12-month post-discharge) and (fixed) explanatory variables were given by treatment arm, baseline values of the variable under investigation (e.g. BMI at pre-randomisation) and randomisation stratifiers. In addition, the models can contain random intercepts for coaches in the ECHO group to allow for correlation in outcomes due to treatment being facilitated by the same coach. The models were used to estimate differences between treatment arms at each time point. Standardised treatment effect estimates were calculated by dividing estimated group differences by the common pre-randomisation standard deviation of the respective outcome.
Outcome variables contained considerable numbers of missing values; see Results section for details. We empirically identified a number of baseline variables that were predictive of missing values in outcome and also found that the primary caregiver not adhering to ECHO (coded ‘1’=completed at least four coaching sessions or read at least 75% the coaching manual, ‘0’=did not complete the intervention) was predictive of loss-to-follow up; see Results section for details. To allow for these processes driving missingness, in addition to allowing randomised group and values of the outcome under investigation at different time points being predictive of missingness, multiple imputation (MI), using chained equations Reference Royston31 with 100 imputations, was implemented. This allowed us to include predictors of missingness (including the post-randomisation variable adherence) in the imputation step without having to condition on these variables in the analyses models. Reference Sterne, White, Carlin, Spratt, Royston and Kenward32
The analyses for continuous caregiver outcomes needed to encompass up to two caregivers per family (a nominated primary and second caregiver). Therefore, the analysis and imputation models described for patients were extended. First, the analysis models contained an additional random intercept that varied at the level of the patient to allow for similar outcomes for caregivers of the same patient. Second, to ensure that correlations were also reflected in the imputed values, imputations were carried out at the level of the patient allowing for two outcome variables – one for the primary caregiver and another for secondary caregiver (set to missing when there was no second caregiver, with resulting imputed values discarded before analysis).
Statistical analyses were carried out in Stata version 11. The user-contributed command ice was used for MI. Reference Royston31
Results
Participant flow
This study represents a collaboration of major UK eating disorder treatment centres and describes a large cross-section of severely ill patients with anorexia nervosa (37% had regular objective binging and 30% had regular vomiting). In total, 178 patients and 268 caregivers were recruited. The CONSORT diagram for the study is shown in Fig. 1.

Fig. 1 Study consort diagram showing participant recruitment, allocation to TAU or TAU augmented with the ECHO intervention. TAU, treatment as usual; BMI, body mass index; DASS, Depression, Anxiety and Stress Scale.
Sample characteristics
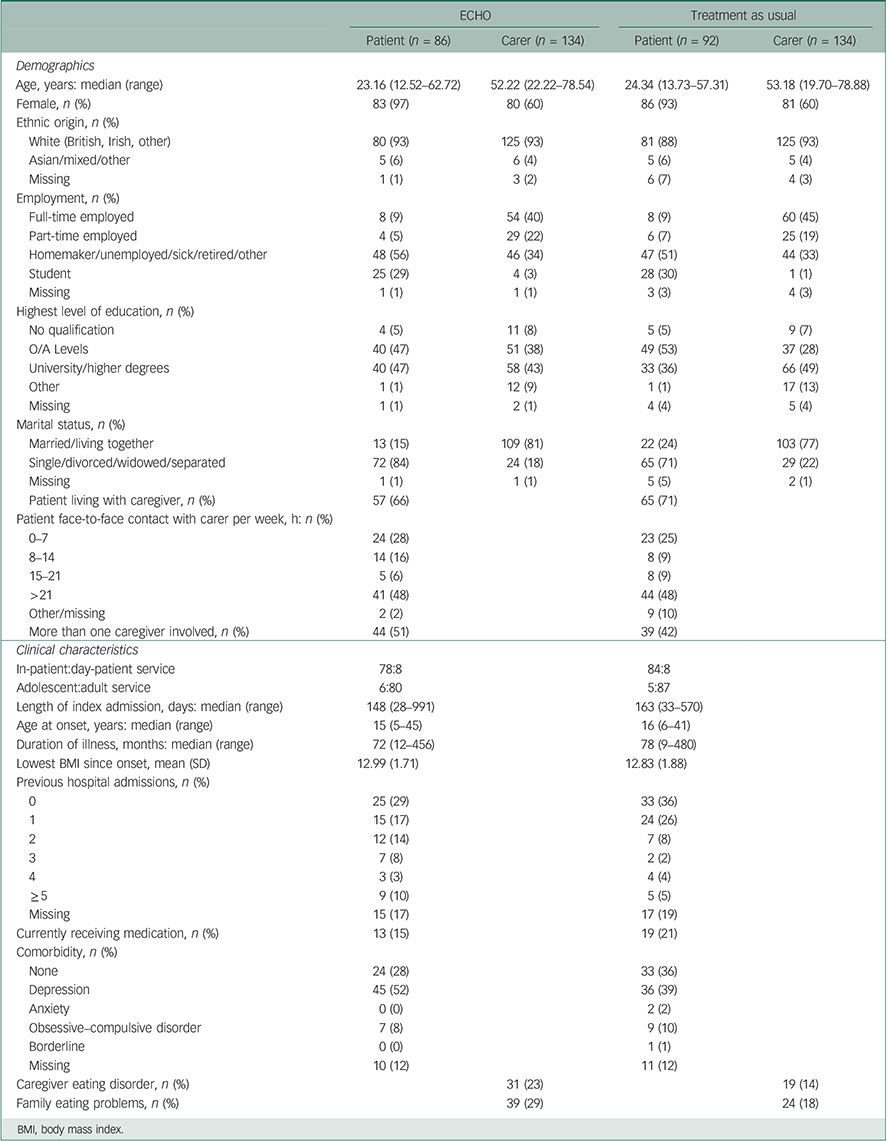
As expected from randomisation, patient and caregiver characteristics were well-balanced across groups (Table 1).
Table 1 Baseline characteristics by treatment group
| ECHO | Treatment as usual | |||
|---|---|---|---|---|
| Patient (n = 86) | Carer (n = 134) | Patient (n = 92) | Carer (n = 134) | |
| Demographics | ||||
| Age, years: median (range) | 23.16 (12.52–62.72) | 52.22 (22.22–78.54) | 24.34 (13.73–57.31) | 53.18 (19.70–78.88) |
| Female, n (%) | 83 (97) | 80 (60) | 86 (93) | 81 (60) |
| Ethnic origin, n (%) | ||||
| White (British, Irish, other) | 80 (93) | 125 (93) | 81 (88) | 125 (93) |
| Asian/mixed/other | 5 (6) | 6 (4) | 5 (6) | 5 (4) |
| Missing | 1 (1) | 3 (2) | 6 (7) | 4 (3) |
| Employment, n (%) | ||||
| Full-time employed | 8 (9) | 54 (40) | 8 (9) | 60 (45) |
| Part-time employed | 4 (5) | 29 (22) | 6 (7) | 25 (19) |
| Homemaker/unemployed/sick/retired/other | 48 (56) | 46 (34) | 47 (51) | 44 (33) |
| Student | 25 (29) | 4 (3) | 28 (30) | 1 (1) |
| Missing | 1 (1) | 1 (1) | 3 (3) | 4 (3) |
| Highest level of education, n (%) | ||||
| No qualification | 4 (5) | 11 (8) | 5 (5) | 9 (7) |
| O/A Levels | 40 (47) | 51 (38) | 49 (53) | 37 (28) |
| University/higher degrees | 40 (47) | 58 (43) | 33 (36) | 66 (49) |
| Other | 1 (1) | 12 (9) | 1 (1) | 17 (13) |
| Missing | 1 (1) | 2 (1) | 4 (4) | 5 (4) |
| Marital status, n (%) | ||||
| Married/living together | 13 (15) | 109 (81) | 22 (24) | 103 (77) |
| Single/divorced/widowed/separated | 72 (84) | 24 (18) | 65 (71) | 29 (22) |
| Missing | 1 (1) | 1 (1) | 5 (5) | 2 (1) |
| Patient living with caregiver, n (%) | 57 (66) | 65 (71) | ||
| Patient face-to-face contact with carer per week, h: n (%) | ||||
| 0–7 | 24 (28) | 23 (25) | ||
| 8–14 | 14 (16) | 8 (9) | ||
| 15–21 | 5 (6) | 8 (9) | ||
| >21 | 41 (48) | 44 (48) | ||
| Other/missing | 2 (2) | 9 (10) | ||
| More than one caregiver involved, n (%) | 44 (51) | 39 (42) | ||
| Clinical characteristics | ||||
| In-patient:day-patient service | 78:8 | 84:8 | ||
| Adolescent:adult service | 6:80 | 5:87 | ||
| Length of index admission, days: median (range) | 148 (28–991) | 163 (33–570) | ||
| Age at onset, years: median (range) | 15 (5–45) | 16 (6–41) | ||
| Duration of illness, months: median (range) | 72 (12–456) | 78 (9–480) | ||
| Lowest BMI since onset, mean (SD) | 12.99 (1.71) | 12.83 (1.88) | ||
| Previous hospital admissions, n (%) | ||||
| 0 | 25 (29) | 33 (36) | ||
| 1 | 15 (17) | 24 (26) | ||
| 2 | 12 (14) | 7 (8) | ||
| 3 | 7 (8) | 2 (2) | ||
| 4 | 3 (3) | 4 (4) | ||
| ≥5 | 9 (10) | 5 (5) | ||
| Missing | 15 (17) | 17 (19) | ||
| Currently receiving medication, n (%) | 13 (15) | 19 (21) | ||
| Comorbidity, n (%) | ||||
| None | 24 (28) | 33 (36) | ||
| Depression | 45 (52) | 36 (39) | ||
| Anxiety | 0 (0) | 2 (2) | ||
| Obsessive–compulsive disorder | 7 (8) | 9 (10) | ||
| Borderline | 0 (0) | 1 (1) | ||
| Missing | 10 (12) | 11 (12) | ||
| Caregiver eating disorder, n (%) | 31 (23) | 19 (14) | ||
| Family eating problems, n (%) | 39 (29) | 24 (18) | ||
BMI, body mass index.
Patients
The descriptive details of the sample and the short-term effects of hospital treatment comparing symptom levels on admission and on discharge from in-patient care have been published. Reference Goddard, Hibbs, Raenker, Salerno, Arcelus and Boughton33
The majority of the patient group (n=178) was significantly underweight (BMI <15 kg/m2) or had medical instability because of electrolyte problems. The mean age was 26 (s.d.=9) years. Eleven cases were from adolescent units; these were approximately equally distributed between the interventions (Table 1). The majority of cases (n=123, 69%) had an illness duration of more than 3 years and 47% (n=83) exceeding 6 years (enduring anorexia nervosa). Of the 11 adolescents: 8 had illness duration <3 years and 2 had illness duration >3 years. (This item was missing on one adolescent.) The median duration of the admission was 153.5 days (range 28–991). One patient remained an in-patient throughout the 2 years of the study.
Caregivers
A total of 268 caregivers (178 primary caregivers, 90 secondary caregivers) were recruited (all were adults: 144 mothers, 81 fathers, 28 partners, 7 siblings, 5 friends, 3 other relatives). The mean number of caregivers per participant was 1.47 (range 1–3); see Table 1 for the number of caregivers involved in each group. The majority (69%) lived with the patient with greater than 21 h of face-to-face contact time per week in 48% cases.
Service characteristics
Only 11 patients were recruited from adolescent units and 16 from day-patient units. As expected, the numbers were equally distributed between trial arms. It is noteworthy that the adolescent units delivered family-based therapy before, during and after the in-patient admission (88.9% of caregivers of patients admitted to hospital at an adolescent in-patient service attended family therapy during hospitalisation, compared with 24.1% of caregivers of patients admitted to hospital to an adult service).
Intervention adherence
Ninety-one caregivers (68% of ECHO arm (15% missing information)) completed 75% of the coaching sessions and the manual.
Important harms or unintended effects
Two patients (one from each trial arm) died during the course of the study.
Outcomes
Fifty (28%) patients at discharge, 57 (32%) at 6-month post-discharge and 66 (37%) at 12 months had missing questionnaire outcome data. The number of caregivers with missing data was 86 (32%) at discharge, 98 (37%) at 6 months and 111 (41%) at 12 months. Service use data (structured interview) were missing from 53 (30%) of patients at 6 months and 55 (31%) at 12 months.
Logistic regression was used to explore the relationships between a dependent variable that represented whether outcome data were present or missing at 12-month post-discharge and a number of baseline demographic and clinical variables, such as participant gender and lowest ever BMI, and also (post-treatment) adherence to ECHO. Any variable that showed a statistically significant association with the dependent variable was included in the imputation step of the MI procedure. Lowest ever BMI was predictive of missingness for patients’ quality of life, eating disorder psychopathology (EDE-Q) and distress (DASS-21); age predicted missingness for patients’ distress. It was also found that non-adherence with allocated therapy was strongly associated with missingness. The associations for caregivers (P-values as low as P<0.001) were stronger than for patients (e.g. P=0.04).
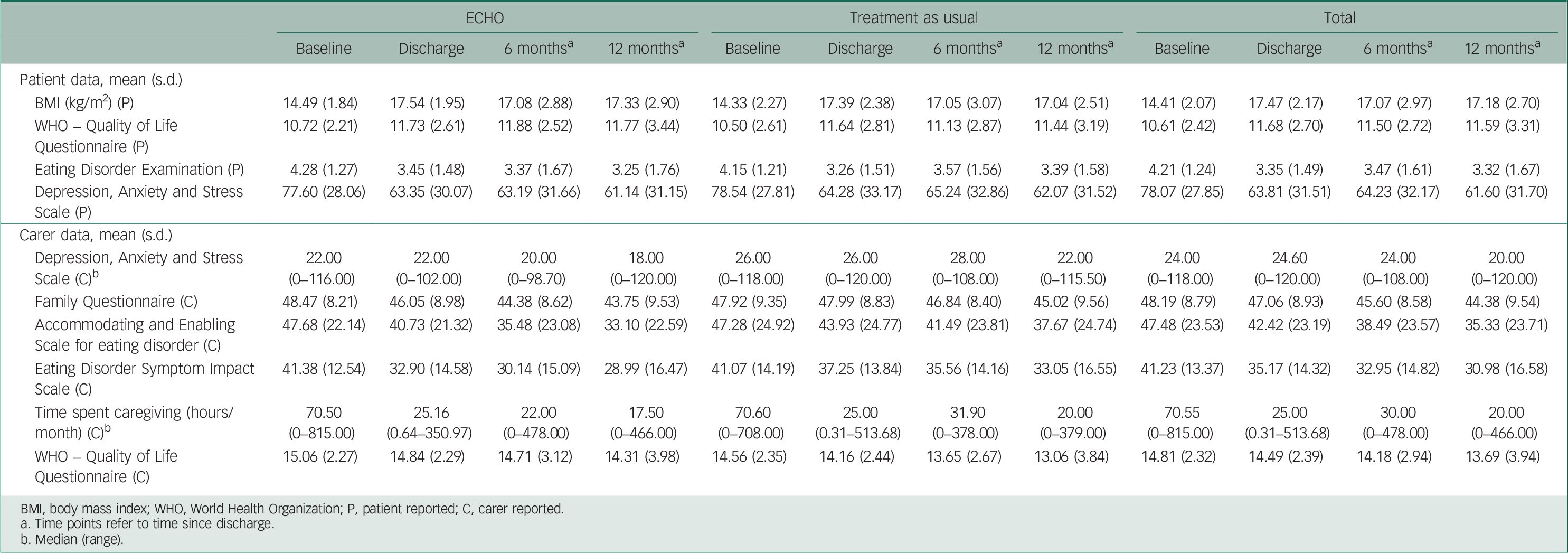
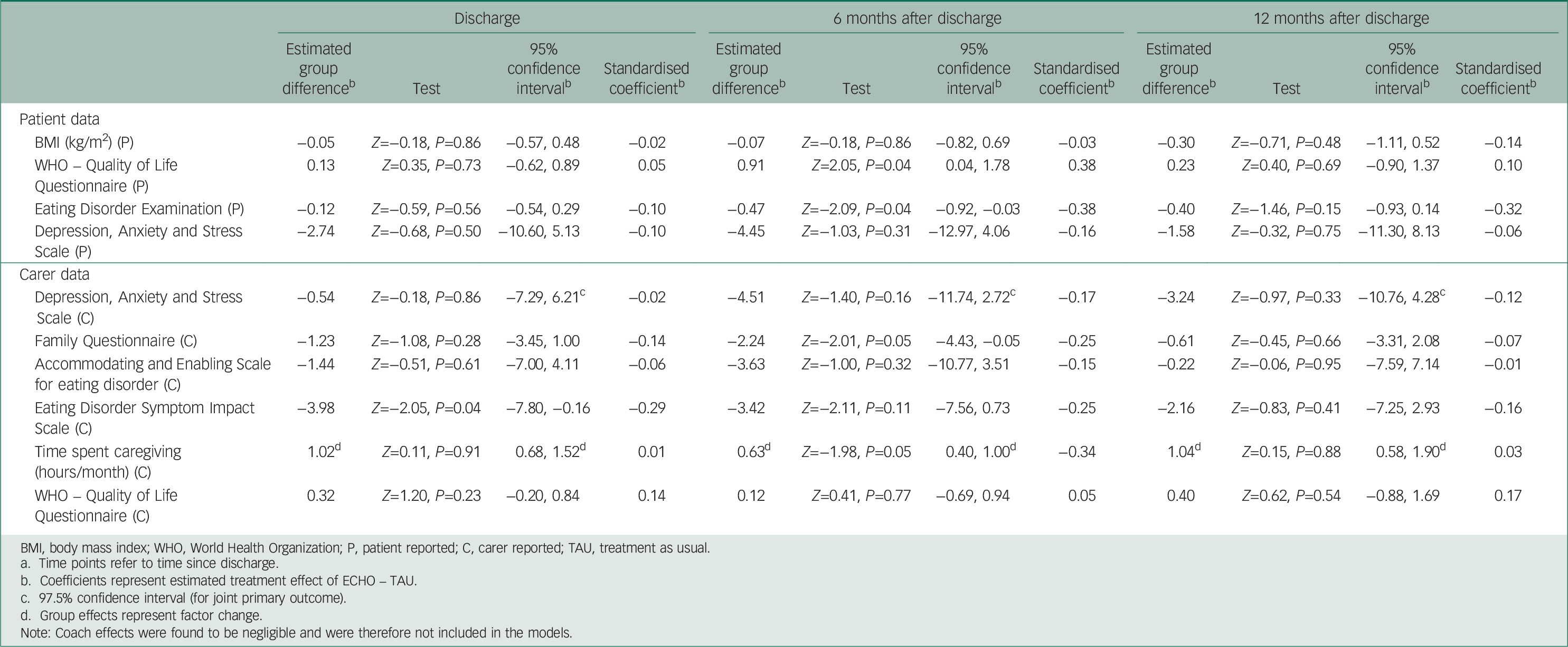
Table 2 summarises the clinical outcomes and Table 3 provides estimated outcome differences between the two treatment arms for both patients and caregivers at all three time points.
Table 2 Summaries of outcome measures by treatment arm and time point
| ECHO | Treatment as usual | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Discharge | 6 months a | 12 months a | Baseline | Discharge | 6 months a | 12 months a | Baseline | Discharge | 6 months a | 12 months a | |
| Patient data, mean (s.d.) | ||||||||||||
| BMI (kg/m2) (P) | 14.49 (1.84) | 17.54 (1.95) | 17.08 (2.88) | 17.33 (2.90) | 14.33 (2.27) | 17.39 (2.38) | 17.05 (3.07) | 17.04 (2.51) | 14.41 (2.07) | 17.47 (2.17) | 17.07 (2.97) | 17.18 (2.70) |
| WHO – Quality of Life Questionnaire (P) | 10.72 (2.21) | 11.73 (2.61) | 11.88 (2.52) | 11.77 (3.44) | 10.50 (2.61) | 11.64 (2.81) | 11.13 (2.87) | 11.44 (3.19) | 10.61 (2.42) | 11.68 (2.70) | 11.50 (2.72) | 11.59 (3.31) |
| Eating Disorder Examination (P) | 4.28 (1.27) | 3.45 (1.48) | 3.37 (1.67) | 3.25 (1.76) | 4.15 (1.21) | 3.26 (1.51) | 3.57 (1.56) | 3.39 (1.58) | 4.21 (1.24) | 3.35 (1.49) | 3.47 (1.61) | 3.32 (1.67) |
| Depression, Anxiety and Stress Scale (P) | 77.60 (28.06) | 63.35 (30.07) | 63.19 (31.66) | 61.14 (31.15) | 78.54 (27.81) | 64.28 (33.17) | 65.24 (32.86) | 62.07 (31.52) | 78.07 (27.85) | 63.81 (31.51) | 64.23 (32.17) | 61.60 (31.70) |
| Carer data, mean (s.d.) | ||||||||||||
| Depression, Anxiety and Stress Scale (C) b | 22.00 (0–116.00) | 22.00 (0–102.00) | 20.00 (0–98.70) | 18.00 (0–120.00) | 26.00 (0–118.00) | 26.00 (0–120.00) | 28.00 (0–108.00) | 22.00 (0–115.50) | 24.00 (0–118.00) | 24.60 (0–120.00) | 24.00 (0–108.00) | 20.00 (0–120.00) |
| Family Questionnaire (C) | 48.47 (8.21) | 46.05 (8.98) | 44.38 (8.62) | 43.75 (9.53) | 47.92 (9.35) | 47.99 (8.83) | 46.84 (8.40) | 45.02 (9.56) | 48.19 (8.79) | 47.06 (8.93) | 45.60 (8.58) | 44.38 (9.54) |
| Accommodating and Enabling Scale for eating disorder (C) | 47.68 (22.14) | 40.73 (21.32) | 35.48 (23.08) | 33.10 (22.59) | 47.28 (24.92) | 43.93 (24.77) | 41.49 (23.81) | 37.67 (24.74) | 47.48 (23.53) | 42.42 (23.19) | 38.49 (23.57) | 35.33 (23.71) |
| Eating Disorder Symptom Impact Scale (C) | 41.38 (12.54) | 32.90 (14.58) | 30.14 (15.09) | 28.99 (16.47) | 41.07 (14.19) | 37.25 (13.84) | 35.56 (14.16) | 33.05 (16.55) | 41.23 (13.37) | 35.17 (14.32) | 32.95 (14.82) | 30.98 (16.58) |
| Time spent caregiving (hours/month) (C) b | 70.50 (0–815.00) | 25.16 (0.64–350.97) | 22.00 (0–478.00) | 17.50 (0–466.00) | 70.60 (0–708.00) | 25.00 (0.31–513.68) | 31.90 (0–378.00) | 20.00 (0–379.00) | 70.55 (0–815.00) | 25.00 (0.31–513.68) | 30.00 (0–478.00) | 20.00 (0–466.00) |
| WHO – Quality of Life Questionnaire (C) | 15.06 (2.27) | 14.84 (2.29) | 14.71 (3.12) | 14.31 (3.98) | 14.56 (2.35) | 14.16 (2.44) | 13.65 (2.67) | 13.06 (3.84) | 14.81 (2.32) | 14.49 (2.39) | 14.18 (2.94) | 13.69 (3.94) |
BMI, body mass index; WHO, World Health Organization; P, patient reported; C, carer reported.
a Time points refer to time since discharge.
b Median (range).
Table 3 Estimated treatment effects on patient and carer and outcome measures at all three post-randomisation time points a
| Discharge | 6 months after discharge | 12 months after discharge | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimated group difference b | Test | 95% confidence interval b | Standardised coefficient b | Estimated group difference b | Test | 95% confidence interval b | Standardised coefficient b | Estimated group difference b | Test | 95% confidence interval b | Standardised coefficient b | |
| Patient data | ||||||||||||
| BMI (kg/m2) (P) | −0.05 | Z=−0.18, P=0.86 | −0.57, 0.48 | −0.02 | −0.07 | Z=−0.18, P=0.86 | −0.82, 0.69 | −0.03 | −0.30 | Z=−0.71, P=0.48 | −1.11, 0.52 | −0.14 |
| WHO – Quality of Life Questionnaire (P) | 0.13 | Z=0.35, P=0.73 | −0.62, 0.89 | 0.05 | 0.91 | Z=2.05, P=0.04 | 0.04, 1.78 | 0.38 | 0.23 | Z=0.40, P=0.69 | −0.90, 1.37 | 0.10 |
| Eating Disorder Examination (P) | −0.12 | Z=−0.59, P=0.56 | −0.54, 0.29 | −0.10 | −0.47 | Z=−2.09, P=0.04 | −0.92, −0.03 | −0.38 | −0.40 | Z=−1.46, P=0.15 | −0.93, 0.14 | −0.32 |
| Depression, Anxiety and Stress Scale (P) | −2.74 | Z=−0.68, P=0.50 | −10.60, 5.13 | −0.10 | −4.45 | Z=−1.03, P=0.31 | −12.97, 4.06 | −0.16 | −1.58 | Z=−0.32, P=0.75 | −11.30, 8.13 | −0.06 |
| Carer data | ||||||||||||
| Depression, Anxiety and Stress Scale (C) | −0.54 | Z=−0.18, P=0.86 | −7.29, 6.21 c | −0.02 | −4.51 | Z=−1.40, P=0.16 | −11.74, 2.72 c | −0.17 | −3.24 | Z=−0.97, P=0.33 | −10.76, 4.28 c | −0.12 |
| Family Questionnaire (C) | −1.23 | Z=−1.08, P=0.28 | −3.45, 1.00 | −0.14 | −2.24 | Z=−2.01, P=0.05 | −4.43, −0.05 | −0.25 | −0.61 | Z=−0.45, P=0.66 | −3.31, 2.08 | −0.07 |
| Accommodating and Enabling Scale for eating disorder (C) | −1.44 | Z=−0.51, P=0.61 | −7.00, 4.11 | −0.06 | −3.63 | Z=−1.00, P=0.32 | −10.77, 3.51 | −0.15 | −0.22 | Z=−0.06, P=0.95 | −7.59, 7.14 | −0.01 |
| Eating Disorder Symptom Impact Scale (C) | −3.98 | Z=−2.05, P=0.04 | −7.80, −0.16 | −0.29 | −3.42 | Z=−2.11, P=0.11 | −7.56, 0.73 | −0.25 | −2.16 | Z=−0.83, P=0.41 | −7.25, 2.93 | −0.16 |
| Time spent caregiving (hours/month) (C) | 1.02 d | Z=0.11, P=0.91 | 0.68, 1.52 d | 0.01 | 0.63 d | Z=−1.98, P=0.05 | 0.40, 1.00 d | −0.34 | 1.04 d | Z=0.15, P=0.88 | 0.58, 1.90 d | 0.03 |
| WHO – Quality of Life Questionnaire (C) | 0.32 | Z=1.20, P=0.23 | −0.20, 0.84 | 0.14 | 0.12 | Z=0.41, P=0.77 | −0.69, 0.94 | 0.05 | 0.40 | Z=0.62, P=0.54 | −0.88, 1.69 | 0.17 |
BMI, body mass index; WHO, World Health Organization; P, patient reported; C, carer reported; TAU, treatment as usual.
a Time points refer to time since discharge.
b Coefficients represent estimated treatment effect of ECHO – TAU.
c 97.5% confidence interval (for joint primary outcome).
d Group effects represent factor change.
Note: Coach effects were found to be negligible and were therefore not included in the models.
A comparison of patient clinical status in the year after hospitalisation between caregivers with and without ECHO intervention
Eating disorder psychopathology (EDE-Q) and quality of life (WHO-Quol) were significantly better in the ECHO group at 6 months effect size (ES)=−0.38 and 0.38 respectively, but these differences were not significant at 12-month post-discharge (Table 3). At 6 months after discharge we found eating disorder psychopathology (EDE-Q) among the ECHO group to be 0.47 points (95% CI 0.03–0.92) less than in the TAU group. At the same time point, we estimated quality of life as measured on the WHO-Quol to be 0.91 points (95% CI 0.04–1.78) higher in the ECHO group compared with the TAU group. Estimated differences in distress and BMI pointed towards a beneficial effect of ECHO, but none of these effects could be shown to be statistically significant.
A comparison of caregivers’ well-being and burden in the year after hospitalisation between caregivers with and without ECHO intervention
Although caregivers in the ECHO group reported fewer symptoms of distress than those in TAU, this was not statistically significant at any time point (Table 3). Caregiving burden (EDSIS), at discharge, was significantly lower in the ECHO group (ES=−0.29). Time spent caregiving (ES=−0.34) and expressed emotion (FQ, ES=−0.25) were significantly lower in the ECHO group at 6 months after discharge. At discharge, we estimated that caregivers’ perceptions of eating disorder burden (EDSIS) in the ECHO group were 3.98 points (95% CI 0.16–7.80) less compared with TAU. At 6 months after discharge we found that time spent caregiving in the ECHO group was 63% (95% CI 40–100) of that spent in the TAU group. At the same time point, we estimated expressed emotion (FQ) in the ECHO group to be 2.24 points (95% CI 0.05–4.43) less on the FQ than in the TAU group. At 12 months, the difference between groups in terms of expressed emotion, accommodating and caregiving time was negligible and there was a small effect in favour of ECHO for mood, burden and quality of life.
A comparison of patient service use between caregivers with and without ECHO intervention
The ECHO group had a slightly shorter median duration of admission (median=148 days, range 28–991) compared with the TAU group (median=163 days, range 33–570) but this was not statistically significant (Mann–Whitney U-test, Z=−0.88, P=0.38). The readmission rate was 27% (n=23) in the ECHO group and 32% (n=29) in the TAU group. Relapse in terms of readmission and/or fall in two BMI points occurred in 43% of the ECHO group and 52% of TAU. The median time to relapse for the ECHO group was 262 days and 240 days for TAU. Survival plots of the time to relapse showed two survival curves that were broadly similar.
A box and whisker plot in Fig. 2 depicts use of beds in the two conditions in the year post-discharge. In the TAU condition, there was a higher bed usage 7–12 months post-discharge than in the ECHO group (Mann–Whitney U-test, Z=−1.97, P=0.049).
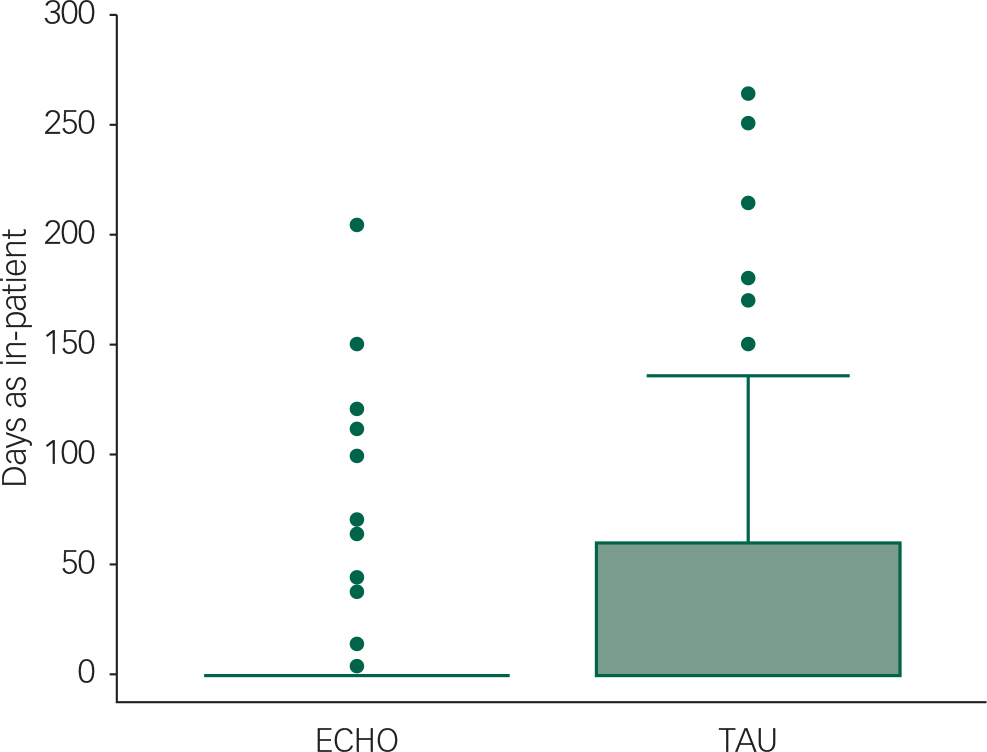
Fig. 2 Box and whisker plot (medians, 75 centile and outliers) for amount of time beds that were occupied in the year following discharge. TAU, treatment as usual.
The summary of bed days, primary care and secondary care appointments in the 6-month and 12-month intervals following discharge are shown in Table 4. There was little difference between the groups in terms of usage of primary and secondary care. Only a small proportion of the sessions in both groups involved the family.
Table 4 Eating disorder-related service use at 6-month and 12-month follow-up post-discharge by treatment group (ECHO experienced carer helping others v. TAU)
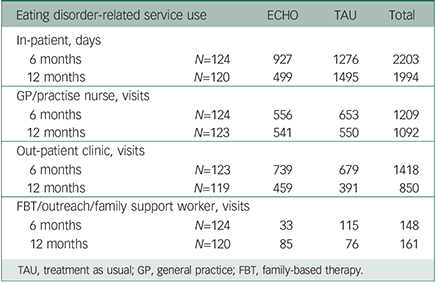
| Eating disorder-related service use | ECHO | TAU | Total | |
|---|---|---|---|---|
| In-patient, days | ||||
| 6 months | N=124 | 927 | 1276 | 2203 |
| 12 months | N=120 | 499 | 1495 | 1994 |
| GP/practise nurse, visits | ||||
| 6 months | N=124 | 556 | 653 | 1209 |
| 12 months | N=123 | 541 | 550 | 1092 |
| Out-patient clinic, visits | ||||
| 6 months | N=123 | 739 | 679 | 1418 |
| 12 months | N=119 | 459 | 391 | 850 |
| FBT/outreach/family support worker, visits | ||||
| 6 months | N=124 | 33 | 115 | 148 |
| 12 months | N=120 | 85 | 76 | 161 |
TAU, treatment as usual; GP, general practice; FBT, family-based therapy.
Discussion
The aim of this study was to evaluate whether giving caregivers a skills training intervention (ECHO) improved both patient and caregiver outcomes in the year following hospital admission. All patients fulfilled the World Health Organization's definition of severe malnutrition 34 (and the majority had an illness duration of more than 3 years). Patients whose caregivers received the ECHO intervention had reduced eating disorder psychopathology (EDE-Q) and improved quality of life (WHO-Quol) at 6 months (both small effects) and reduced in-patient bed days (7–12 months post-discharge). Estimated differences in distress and BMI pointed towards a beneficial effect of ECHO, but none of these effects could be shown to be statistically significant. Caregivers in the ECHO group had a small/moderate reduction in caregiver burden and reduced expressed emotion and a greater reduction in their time caregiving 6 months after discharge but these changes were diminished at 12 months. We did not find statistically significant effects of ECHO in terms of our distal primary outcomes, patient relapse and caregiver distress, although differences were in the anticipated direction. The size of the change effects for all secondary outcomes for both caregiver and patient were small but all favoured ECHO. Fewer bed days were used in the ECHO group.
The impact of the intervention on caregiver outcomes was limited to the first 6 months after discharge. This suggests that further booster sessions of family skills training throughout the year following admission or joint caregiver/patient sessions may be needed. It is noteworthy that the reduced bed use is not at the cost of greater burden and time caregiving from caregivers. Indeed the opposite effect occurred.
Interestingly, the size of the effect in terms of improvement of patient symptomatology remained present at 1 year suggesting that the indirect impact on patients may be more sustained despite less in-patient use. A 2-year follow-up of these patients that is in progress will be of interest to validate this conclusion.
The qualitative feedback (previously reported) also indicates that both patient and caregiver found the intervention helpful. Reference Macdonald, Rhind, Hibbs, Goddard, Raenker and Todd35 Patients reported changes in their caregivers that include a greater understanding and awareness of the illness and improved coping abilities, better communication and reduced anxiety. Caregivers themselves noted improvements in their caregiving skills.
Strengths
This study used the strengths of a pragmatic randomised controlled design set within the majority of specialised eating disorder centres in England and so is relevant to current NHS England practice. This is one of the largest RCT studies within an in-patient setting and unique in considering the caregiver perspective. We added the intervention to TAU for pragmatic and economic reasons. We obtained information from three sources, patients, caregivers and services. The intervention was designed to be easy to disseminate and uses many principles advocated for a global application. Reference Fairburn and Patel36 As such, we used ‘task sharing’ with people with lived experience as we had found this expertise valued in our pilot studies. A small proportion of postgraduate psychologists were trained to increase the capacity to deliver the intervention. In a previously reported study, we found satisfactory levels of treatment fidelity in both groups with these different background experiences. Reference Macdonald, Hibbs, Rhind, Harrison, Goddard and Raenker21 Thus, given the low cost of the intervention, and the reduction in bed use (cost £470/day) and the potential for easy scalability and reach, we consider that this intervention is clinically relevant.
Limitations
Because of the research requirements (the agreement to involve caregivers), the sample is not necessarily representative of the intended target population. This might affect generalisability although in practice, because these materials are delivered directly to the caregiver, they could be part of standard information offered to caregivers in order to fulfil in-patient quality standards. Reference Cresswell, Beavon and Robinson23 In hindsight, a more eating disorder-related specific outcome such as caregiver burden or quality of life rather than caregiver distress may be more appropriate as the primary outcome for caregivers. Also in hindsight, the use of a binary/time to event outcome ‘relapse’ such as our primary patient outcome was not useful for this patient group most of whom continue to meet diagnostic criteria at discharge. Other outcomes such as number of bed days, quality of life, BMI and/or the level of psychopathology would be clinically more meaningful.
Although there are standard quality criteria for in-patient care, the treatment ethos does vary between services. For example, some services involve caregivers more than others. We found that very little family support was offered as part of aftercare (on average 1 session per case). It is noteworthy that the adolescent units delivered family-based therapy before, during and after the in-patient admission. Reference Macdonald, Hibbs, Rhind, Harrison, Goddard and Raenker21 It is possible that this may have diluted the effect for these 11 families or it may even been harmful if the messages delivered were contradictory. Work is in progress to examine this possibility. This variability within the TAU condition may have decreased the size of group effect but there would be no bias in its estimation as the trial design was stratified by site. Further work to examine the factors that may influence the overall outcome will be of interest.
Clinical implications
A low-intensity skills-sharing intervention for the caregivers of patients with anorexia nervosa admitted for hospital care produced a small decrease in caregiver burden, expressed emotion and time spent caregiving at 6-month post-discharge but with less of an effect later. There was a small but sustained improvement in in-patients’ quality of life and clinical symptoms. In-patient bed use 6–12 months after admission was reduced. This study illustrates how involving the family during in-patient care for adults as well as children can have benefits for both patient and caregiver. However, this form of augmentation of skills sharing for caregivers probably needs to be continued during the aftercare maintenance phase, particularly as caregivers may be the primary source of support for the individual during this time. The intervention has the potential to be easily disseminated and there may be a synergy in combining this with a skills-based intervention for patients themselves. Further work investigating the optimisation of in-patient care and after care for people with severe enduring anorexia nervosa is needed. It is noteworthy that in current NHS practice, in-patient care is a palliative intervention, only partially restoring weight. Although on average, the treatment gains are maintained in the year post-discharge, most patients remain severely ill. This poor outcome and the two deaths within the study highlight the need for better treatment for this patient group.
Acknowledgements
We thank all families who participated and to the staff on the in-patient units that facilitated the study. We would also like to thank the Mental Health Research Network whose CSOs facilitated recruitment.
This article presents independent research commissioned by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research scheme (reference number RP-PG-0606-1043). Janet Treasure, Ulrike Schmidt and Sabine Landau received salary support from the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Our great thanks go to our experienced coaches who gave of their time and expertise to be trained in and to deliver the coaching: SnitaAhir-Knight, Leigh Best, Yvonne Boughton, Russell Cooper, Jocelyn de Guzman, Suzi Doyle, Amy Harrison, Jenny Langley. Melinda Waldrum, Esther Ritchie, Yvonne Round, Marjenah Shahabi, Jilly Shipway and Clare Walker. This paper is in memory of Charlotte Bevan, a mother caregiver who strove to improve the support and skills of caregivers.
We also thank the principal investigators who are involved in the recruitment of participants at in-patient/day-patient sites: L. Whitehead, Cotswold House, Oxford; D. Robertson, Barberry Unit, Birmingham; A. Ayton, Darwin Centre, North Staffordshire; S. Sharma, Cheadle Royal Hospital, Manchester; K. Moore, Kinver Centre, South Staffordshire; B. Bamford, St George's, London; N. Boughton, Cotswold House, Oxford; F. Connan, St Vincent's Clinic, London; K. Goss, Coventry; B. Laszlo, Haldon Unit, Exeter; C. Schreiber-Kounine, STEPS, Bristol; H. Lacey, St George's, London.
Appendix: NHS England sites
-
1. Barberry Unit, Birmingham
-
2. Bethlem Royal Hospital, London
-
3. Brandon Unit, Leicester
-
4. Cheadle Royal Hospital, Manchester
-
5. Cotswold House, Marlborough
-
6. Cotswold House, Oxford
-
7. Coventry
-
8. Darwin Centre, North Staffordshire
-
9. Haldon Unit, Exeter
-
10. Highfield Unit
-
11. Kinver Centre, South Staffordshire
-
12. Seacroft Hospital, LPFT
-
13. STEPS, Bristol
-
14. St George's, London
-
15. Vincent's Square, London










eLetters
No eLetters have been published for this article.