All human tissues are in a constant state of remodelling, which is regulated by the balance between tissue protein synthesis and breakdown rates(Reference van Dijk, Horstman and Smeets1,Reference Burd, Hamer and Pennings2) . Food ingestion forms a major anabolic stimulus for skeletal muscle tissue(Reference Groen, Horstman and Hamer3,Reference Volpi, Kobayashi and Sheffield-Moore4) . Protein ingestion delivers amino acids that stimulate protein synthesis (e.g. leucine) and are used as precursors to support de novo protein synthesis. Various dietary factors modulate the protein synthetic response to protein ingestion, including the amount and quality of the ingested protein(Reference Trommelen, Betz and van Loon5–Reference Tang, Moore and Kujbida7). The capacity of dietary protein to stimulate protein synthesis largely depends on the digestion and amino acid absorption kinetics and amino acid composition of the ingested protein(Reference Pennings, Boirie and Senden8,Reference Wolfe, Rutherfurd and Kim9) .
Post-prandial protein handling encompasses the various processes by which the ingested protein is digested, absorbed and metabolised in tissues (e.g. protein synthesis and oxidation). Stable isotope-labelled amino acid methodologies are commonly applied to assess the individual aspects of protein metabolism in vivo in human subjects(Reference Volpi, Mittendorfer and Wolf10–Reference Hinde, O'Leary and Greeves14). For example, the infusion of stable isotope-labelled amino acids is commonly applied to assess basal, post-absorptive or post-prandial muscle protein synthesis rates. However, this approach does not allow the assessment of dietary protein digestion and amino acid absorption kinetics and the subsequent impact on whole-body protein synthesis, breakdown or amino acid oxidation.
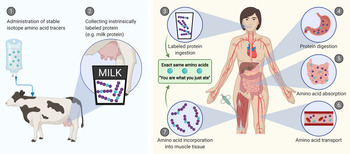
A more comprehensive assessment of post-prandial protein handling in vivo in human subjects can be achieved by combining the infusion of stable isotope-labelled amino acids with the ingestion of intrinsically labelled protein and frequent collection of blood and muscle samples. Intrinsically labelled milk protein was first obtained by Boirie et al. via infusing a lactating cow with l-[1-13C]-leucine, which resulted in the incorporation of l-[1-13C]-leucine into the milk protein matrix(Reference Boirie, Fauquant and Rulquin15). The intrinsically labelled milk was collected and subsequently ingested by human test participants. Blood sample collection and analysis allows the in vivo assessment of the post-prandial appearance rate of milk protein-derived l-[1-13C]-leucine into the circulation(Reference Boirie, Dangin and Gachon16). We and others have further improved this approach to assess both post-prandial protein handling and subsequent metabolism(Reference Pennings, Pellikaan and Senden17–Reference Reitelseder, Tranberg and Agergaard20). This approach (Fig. 1) now allows the assessment of (1) protein digestion and amino acid absorption kinetics (total amino acid rate of appearance, protein-derived amino acid rate of appearance, endogenous amino acid rate of appearance and total amino acid rate of disappearance into tissues), (2) whole-body protein metabolism (whole-body protein synthesis, breakdown and oxidation rates and net protein balance) and (3) post-prandial skeletal muscle metabolism (muscle protein fractional synthesis rates and specific dietary protein-derived amino acid incorporation into muscle protein). The purpose of this review is to provide an overview of the various aspects of post-prandial protein handling and metabolism with a focus on insights obtained from studies that have applied intrinsically labelled protein under a variety of conditions in different populations.

Fig. 1. Schematic representation of the production of intrinsically labelled protein sources to assess various aspects of post-prandial protein handling. Here, the production of intrinsically labelled milk: (1) stable isotope amino acid tracers are administered to lactating cows, (2) the cow produces milk with the amino acid tracer incorporated into the milk protein matrix. Application of intrinsically labelled protein: (3) the collected intrinsically labelled milk protein is consumed by participants, (4) dietary protein is digested into peptides and amino acids, (5) dietary protein-derived amino acids, di- and tri-peptides are taken up from the gastrointestinal lumen by enterocytes, (6) dietary protein-derived amino acids are released into the circulation and (7) dietary protein-derived amino acids are taken up and incorporated into tissues, such as skeletal muscle.
Dietary protein-derived amino acid release into the circulation
Dietary protein-derived amino acid release into the circulation is preceded by a series of complex processes, including mastication, gastric mixing, gastric emptying into the intestine, protein cleavage into amino acids, di- and tri-peptides, transport into the enterocytes, amino acid extraction by splanchnic tissues and the release of the remaining dietary protein-derived amino acids into the circulation. The rise in circulating plasma amino acid concentrations is often used as a proxy of dietary protein digestion and amino acid absorption(Reference Trommelen, Weijzen and van Kranenburg21–Reference Traylor, Gorissen and Hopper23). However, changes in plasma amino acid concentrations do not necessarily reflect the mere appearance rate of exogenous (dietary protein-derived) amino acids as they are also impacted by changes in the release of amino acids originating from various tissues and the (rapid) uptake of amino acids in these tissues. The post-prandial rise in circulating plasma amino acid concentrations reflects dietary protein digestion and amino acid absorption as well as the uptake (synthesis and oxidation) and release (breakdown) of amino acids by other tissues. Therefore, a more direct assessment of the rate of protein-derived exogenous amino acid release into the circulation (EXORa) is required to determine to what extent the ingested protein is digested, absorbed and subsequently released as amino acids into the circulation. To assess the EXORa in vivo in human subjects, an intravenous stable isotope amino acid infusion is combined with the ingestion of intrinsically labelled protein in which the same amino acid with a different isotope label is incorporated within the protein matrix(Reference Trommelen, Holwerda and Nyakayiru24). The labelled dietary protein-derived amino acids are digested and absorbed identically to unlabelled amino acids, allowing us to quantify the EXORa of the dietary protein. By calculating the incremental area under the curve of exogenous amino acid rate of appearance, the total amount of dietary protein-derived amino acids appearing in the circulation can be calculated and/or expressed as a percentage of the amount of the specific amino acid provided within the ingested protein.
Factors affecting dietary protein-derived amino acid release into the circulation
The combination of intrinsically labelled protein with intravenous stable isotope amino acid infusion has been applied to identify different dietary factors that modulate EXORa. The ingestion of greater amounts of protein results in a greater EXORa up to (at least) the ingestion of as much as 45 g protein(Reference Churchward-Venne, Pinckaers and Smeets6,Reference Holwerda, Paulussen and Overkamp25–Reference Kouw, Holwerda and Trommelen27) . Different types of proteins also modulate EXORa. For instance, casein protein ingestion results in a relatively lower but more prolonged EXORa, whereas whey protein ingestion results in a relatively rapid, but more transient, increase in EXORa(Reference Pennings, Boirie and Senden8,Reference Boirie, Dangin and Gachon16,Reference Gorissen, Trommelen and Kouw26) . This difference has been attributed to the clotting of micellar casein protein in the acidic environment of the stomach, thereby delaying gastric emptying and subsequent protein digestion(Reference Ye, Cui and Dalgleish28). Milk protein ingestion results in an intermediate EXORa when compared to whey and casein protein ingestion(Reference Gorissen, Trommelen and Kouw26). Milk protein ingestion is followed by a lower EXORa when compared to the ingestion of an equivalent amount of minced (cooked) beef(Reference Burd, Gorissen and van Vliet29). The digestion and absorption kinetics of ingested protein are not only dependent on the inherent properties of the protein, but are also modulated by protein processing and/or meal preparation. In this regard, the ingestion of a protein hydrolysate results in an accelerated EXORa when compared with the ingestion of the same, intact protein(Reference Pennings, Boirie and Senden8,Reference Koopman, Crombach and Gijsen30) . In a similar context, the ingestion of minced (cooked) meat results in a higher EXORa when compared to the ingestion of (cooked) beef steak(Reference Pennings, Groen and van Dijk31). Furthermore, co-ingestion of other macronutrients impacts the EXORa following protein ingestion. For instance, carbohydrate co-ingestion attenuates the EXORa(Reference Gorissen, Burd and Hamer32). In agreement, the EXORa of casein is attenuated when ingested within a carbohydrate-containing milk matrix(Reference Churchward-Venne, Snijders and Linkens33). However, the co-ingestion of milk fat within a casein protein beverage does not impact EXORa(Reference Gorissen, Burd and Kramer34). In contrast, egg-white consumption resulted in a more rapid EXORa when compared to the ingestion of an isonitrogenous amount of whole egg that contained substantially more fat(Reference van Vliet, Shy and Abou Sawan35). The discrepancy in the impact of fat co-ingestion on EXORa is possibly due to how fat interacts with protein within the food matrix. For instance, co-ingested fat likely separates from protein within the stomach following consumption as a beverage, whereas fat and proteins may remain homogenous in a matrix following whole egg ingestion, facilitating greater macronutrient interaction(Reference Edelbroek, Horowitz and Maddox36).
In addition to dietary factors, the EXORa is modulated by physiological conditions. Protein ingestion in older individuals results in lower EXORa when compared to the ingestion of the same amount of protein by younger adults(Reference Gorissen, Trommelen and Kouw26,Reference Gorissen, Burd and Hamer32,Reference Groen, Horstman and Hamer37) , and EXORa is lower in obese v. lean individuals(Reference Kouw, van Dijk and Horstman38). Furthermore, EXORa seems to be attenuated during recovery from resistance-, but not endurance-type exercise(Reference van Wijck, Pennings and van Bijnen39,Reference Mazzulla, Parel and Beals40) . Finally, maintenance haemodialysis patients display a lower EXORa following egg ingestion when compared to healthy controls(Reference van Vliet, Skinner and Beals41). Taken together, it is clear that dietary protein-derived amino acid release is strongly modulated by several factors related to the composition and preparation of the ingested meal along with other physiological factors.
Post-prandial whole-body protein metabolism
The assessment of whole-body protein metabolism (i.e. protein synthesis, breakdown and amino acid oxidation rates) is based on the rates of amino acid appearance in and disappearance from the circulation. In a fasted state, protein breakdown is the only source of amino acid release into the circulation and can be quantified based on a continuous intravenous stable isotope amino acid infusion. In the fed state, amino acids released in the circulation can be from endogenous and exogenous origin as a result of tissue protein breakdown and protein ingestion, respectively. The application of intrinsically labelled protein enables us to differentiate between dietary protein-derived amino acids and endogenous amino acids release. Therefore, the combined application of intravenous stable isotope amino acid infusions with the ingestion of intrinsically labelled protein allows us to accurately assess post-prandial protein handling.
Factors affecting post-prandial whole-body protein metabolism
The combined application of continuous intravenous infusion of stable isotope amino acids and ingestion of intrinsically labelled protein has been used to establish that intrinsically labelled milk protein ingestion stimulates whole-body protein synthesis rates, attenuates whole-body protein breakdown rates and improves whole-body protein net balance in a dose-dependent manner up to doses of at least 45 g(Reference Churchward-Venne, Pinckaers and Smeets6,Reference Holwerda, Paulussen and Overkamp25,Reference Kouw, Holwerda and Trommelen27) . This response can be modulated by the type of protein ingested. For example, the ingestion of intrinsically labelled minced beef has been shown to improve whole-body net protein balance to a greater extent than ingesting the same beef in the form of an intrinsically labelled steak(Reference Pennings, Groen and van Dijk31). Carbohydrate co-ingestion has been shown to further reduce whole-body protein breakdown and amino acid oxidation(Reference Gorissen, Burd and Hamer32). This may in part be attributed to its insulinotropic properties(Reference Groen, Horstman and Hamer37).
Apart from nutritional factors, physiological factors also modulate the effect of protein ingestion on whole-body protein metabolism. Resistance- and endurance-type exercises appear to have little-to-no impact on whole-body net protein balance(Reference Mazzulla, Parel and Beals40,Reference Trommelen, Holwerda and Kouw42,Reference Holwerda, Kouw and Trommelen43) . The post-prandial reduction in whole-body protein breakdown rates is attenuated in older adults when compared to younger adults(Reference Gorissen, Burd and Hamer32). In line, older adults display less of a post-prandial increase in whole-body net balance when compared to younger adults(Reference Groen, Horstman and Hamer37). Finally, obese individuals seem to display less of a post-prandial increase in whole-body net protein balance when compared to lean individuals following ingestion of 25 g protein. The latter may be explained by the lower dosage when expressed per kg body mass, resulting in less of an increase in post-prandial amino acid availability expressed per kg body mass(Reference Kouw, van Dijk and Horstman38). Although several factors have been shown to impact whole-body net protein balance, post-prandial changes in plasma amino acid availability seem to represent the main determinant defining the impact of feeding on the post-prandial whole-body net protein balance.
Post-prandial muscle protein metabolism
The assessment of post-prandial whole-body protein metabolism allows a holistic view of the anabolic response of the sum of all tissues to anabolic stimuli, such as food intake and physical activity. However, different organs and organ regions have substantially different tissue protein synthesis rates and may respond quite differently to various stimuli(Reference van Dijk, Horstman and Smeets1,Reference Smeets, Horstman and Vles44,Reference Smeets, Horstman and Schijns45) . Therefore, whole-body metabolic responses may not represent the response of a specific tissue of interest. To directly assess protein synthesis rates in a specific tissue, a continuous intravenous infusion of stable isotope amino acids is combined with tissue biopsy sampling, using the precursor-product method(Reference Wilkinson46). The precursor-product method is most commonly performed to determine fractional protein synthesis rates in skeletal muscle tissue, which represents the organ containing the largest proportion of total body protein due to its extensive mass (40 % of whole-body protein pool). Furthermore, biopsy sampling from skeletal muscle tissue is relatively easy when compared to tissue collection from other organs(Reference Tarnopolsky, Pearce and Smith47). Furthermore, skeletal muscle tissue is highly adaptive in response to physical activity and nutrition(Reference Trommelen, Betz and van Loon5). Given that the stimulation of skeletal muscle protein synthesis is a key regulatory factor for muscle mass and function, the precursor-product method has been applied extensively to study skeletal muscle metabolism in various populations and conditions. Dietary and physiological factors affecting muscle protein synthesis have been the topic of several recent reviews(Reference Trommelen, Betz and van Loon5,Reference Gorissen and Witard48,Reference Wolfe49) .
The use of intrinsically labelled protein to quantify post-prandial muscle protein synthesis
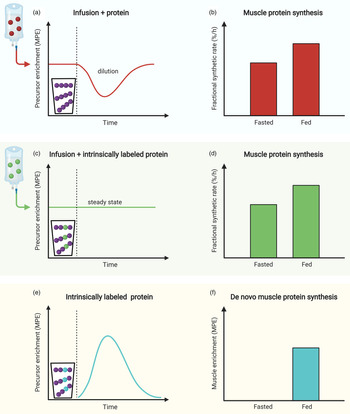
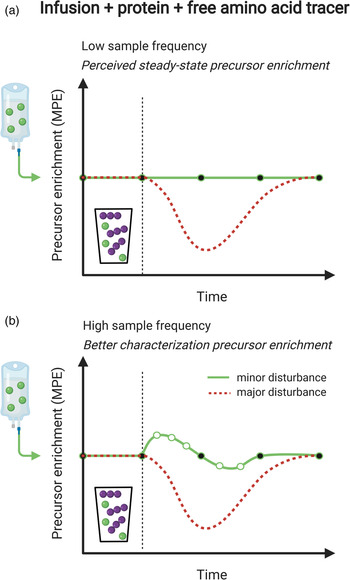
Measurement of the muscle protein synthetic response following food (protein) intake is of great importance, but accurate quantification can be challenging. An accurate assessment of muscle protein synthesis requires a steady state in the stable isotope-labelled amino acid precursor pool (i.e. in the plasma or muscle-free amino acid pools). However, protein ingestion is followed by the release of unlabelled amino acids into the circulation, which destabilises the precursor pool(s) by rapid dilution followed by a gradual return to steady-state conditions (Fig. 2a)(Reference Burd, Cermak and Kouw50). Destabilisation of the precursor pools during the post-prandial period may lower the capacity to detect small changes in muscle protein synthesis rates following food intake (Fig. 2b)(Reference Burd, Cermak and Kouw50). However, such a destabilisation in the precursor pools can be prevented by the application of intrinsically labelled protein, in which the labelled amino acid in the protein matches the labelled amino acid that is intravenously infused (Fig. 2c). To avoid precursor destabilisation, the labelled amino acid enrichment in the dietary protein must be comparable to the plasma enrichment that will be reached during intravenous-labelled amino acid infusion. Upon protein ingestion, the dietary protein-derived labelled amino acids are digested and absorbed identically to the unlabelled protein-derived amino acids. As a result, the labelled and unlabelled amino acids are uniformly delivered into the plasma and peripheral tissues, which allows maintenance of a steady-state isotope tracer enrichment of the precursor pool(s). This allows for a more sensitive assessment of the muscle protein synthetic response to feeding (Fig. 2d). Although the use of intrinsically labelled protein forms the preferred approach to avoid precursor pool destabilisation during the measurement of post-prandial muscle protein synthesis rates, the co-ingestion of labelled free amino acids with dietary protein represents a less costly alternative. However, free amino acids are much more rapidly absorbed in comparison with intact protein. Therefore, free amino acid co-ingestion may result in a transient increase in precursor enrichment during the early post-prandial period, before eventually returning to steady-state conditions (Fig. 3a). When labelled free amino acids are co-ingested, more frequent plasma sampling should be applied to confirm and appropriately characterise the destabilisation of the post-prandial isotope tracer precursor pool enrichment (Fig. 3b).

Fig. 2. Schematic representation of the use of intrinsically labelled dietary protein to accurately quantify post-prandial muscle protein synthesis rates as well as de novo muscle protein synthesis. (a) and (b): When protein is ingested, the steady-state precursor enrichment obtained by intravenous infusion of stable isotope amino acid tracers is diluted. This dilution of the precursor pool enrichment may compromise the ability to quantify the muscle protein synthetic response to feeding. (c) and (d): When ingesting intrinsically labelled protein, in which the labelled amino acid in the protein matches the labelled amino acid that is intravenously infused, dilution of the precursor pool can be prevented and tracer steady-state may be maintained. This allows for a more accurate measurement of the muscle protein synthetic response to feeding. (e) and (f): The presence of a labelled amino acid (that is not infused) in the dietary protein allows for the assessment of the incorporation of dietary protein-derived amino acids into muscle tissue protein (i.e. de novo muscle protein synthesis).

Fig. 3. Schematic representation of the co-ingestion of labelled free amino acids (corresponding to intravenously infused labelled amino acids) with dietary protein as a means to maintain precursor pool enrichments for the accurate measurement of post-prandial muscle protein synthesis rates. (a): The red dotted line represents dilution of the (infused) labelled amino acid precursor pool following the ingestion of dietary protein. Co-ingestion of the same labelled amino acid allows maintenance of isotope tracer steady-state conditions. (b): Ingestion of a free, crystalline (isotope-labelled) amino acid will be more rapidly absorbed when compared to the ingestion of the same (isotope-labelled) amino acid when it is incorporated into an intact protein. Therefore, co-ingestion of a labelled amino acid cannot prevent disruption of the isotope tracer steady-state. Therefore, it is at all times important to correctly assess changes in precursor pool enrichments over time, which requires a high blood or tissue sampling frequency.
De novo muscle protein synthesis
In addition to facilitating the assessment of post-prandial muscle protein synthesis rates, intrinsically labelled protein allows for the assessment of the incorporation of dietary protein-derived amino acids into muscle tissue protein (i.e. de novo muscle protein synthesis; Fig. 2f). This measurement requires the intrinsically labelled protein to be highly enriched with an amino acid label that is different from the one infused intravenously. Intrinsically (doubly) labelled protein containing two different labelled amino acids (one matching the amino acid tracer applied in the infusate) allows the simultaneous assessment of the incorporation of dietary protein-derived amino acids into muscle proteins and post-prandial fractional muscle protein synthesis rates. The direct measurement of the incorporation of protein-derived amino acids into skeletal muscle protein encompasses all upstream processes of dietary protein handling, including protein digestion and amino acid absorption, delivery to skeletal muscle tissue, transport into the muscle tissue and utilisation as a precursor for de novo muscle protein synthesis. As such, the direct assessment of the incorporation of dietary protein-derived amino acids in tissue protein indicates to what extent the ingested dietary protein is delivered to and utilised within in the tissue to support its remodelling. Therefore, highly enriched intrinsically labelled protein can provide valuable information on how dietary protein is utilised by tissues in a variety of experimental settings.
Factors affecting the incorporation of dietary protein-derived amino acids into muscle protein
The use of highly enriched intrinsically labelled protein has consistently shown that dietary protein-derived amino acids are utilised for de novo muscle protein synthesis across a wide variety of populations and can be modulated by various dietary and physiological conditions(Reference Groen, Horstman and Hamer3,Reference Kouw, Holwerda and Trommelen27,Reference Trommelen, Kouw and Holwerda51,Reference Fuchs, Kouw and Churchward-Venne52) . Ingested protein delivers amino acids as precursors for de novo muscle protein synthesis in mixed muscle protein(Reference Groen, Horstman and Hamer3), and has also been detected in various isolated muscle protein fractions, such as myofibrillar(Reference Trommelen, Kouw and Holwerda51), mitochondrial(Reference Churchward-Venne, Pinckaers and Smeets6) and connective tissue(Reference Trommelen, Holwerda and Senden53) proteins. Our research group has applied intrinsically labelled protein to demonstrate that the ingestion of 20 g micellar casein protein results in the incorporation of approximately 2 g (about 10 %) dietary protein-derived amino acids into muscle tissue protein during a 5 h post-prandial period(Reference Groen, Horstman and Hamer3). The observation that dietary protein-derived amino acids are directly incorporated into skeletal muscle protein within the hours following meal ingestion provided us with the evidence that ‘you are what you just ate’(Reference Groen, Horstman and Hamer3). More recent study has demonstrated that the ingestion of greater amounts of dietary protein results in greater amounts of dietary protein-derived amino acids being incorporated into skeletal muscle protein, with no indication of an upper limit up to the ingestion of a dose of 45 g protein(Reference Churchward-Venne, Pinckaers and Smeets6,Reference Holwerda, Paulussen and Overkamp25,Reference Kouw, Holwerda and Trommelen27) . However, aside from the amount of ingested protein, the type of ingested protein has also been shown to impact dietary protein-derived amino acid incorporation into skeletal muscle protein. In particular, whey protein has been shown to result in a greater incorporation of dietary protein-derived amino acid into skeletal muscle proteins when compared to micellar casein and hydrolysed micellar casein(Reference Pennings, Boirie and Senden8). This may be explained by the more rapid release of protein-derived amino acids following whey v. casein ingestion and the higher leucine content of whey protein(Reference Wall, Hamer and de Lange54,Reference Holwerda, Paulussen and Overkamp55) .
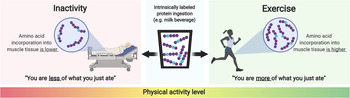
Besides dietary factors, physiological factors have also been shown to impact the incorporation of dietary protein-derived amino acids into muscle tissue. For instance, prior exercise and neuromuscular electrical stimulation have been shown to result in greater de novo muscle protein synthesis(Reference Trommelen, Holwerda and Kouw42,Reference Holwerda, Kouw and Trommelen43,Reference Wall, Burd and Franssen56,Reference Pennings, Koopman and Beelen57) . Resistance-type exercise enhances the capacity to incorporate dietary protein-derived amino acids into muscle tissue for at least 12 h(Reference Wall, Burd and Franssen56). However, post-exercise cooling reduces the incorporation of dietary protein-derived amino acids following post-exercise meal intake(Reference Fuchs, Kouw and Churchward-Venne52). Lastly, short-term muscle disuse results in lower de novo muscle protein synthesis(Reference Wall, Dirks and Snijders58) (Fig. 4). Therefore, it is evident that physical (in)activity is an important factor modulating the capacity to utilise dietary protein-derived amino acids as precursors for de novo muscle protein synthesis.

Fig. 4. Schematic representation of the impact of physical (in)activity on the incorporation of dietary protein-derived amino acids into skeletal muscle protein.
Simultaneous assessment of multiple aspects of post-prandial protein handling
A benefit of using a comprehensive oral-intravenous tracer approach is that various aspects of post-prandial protein handling can be assessed within the same experiment under identical experimental conditions. It is evident that protein digestion and amino acid absorption modulate most of the downstream elements of post-prandial protein handling. Dietary protein-derived amino acid release into the circulation appears to be the main determinant of whole-body protein balance and dietary protein-derived amino acid incorporation into muscle tissue. All three have demonstrated a dose–response relationship with no sign of an upper limit with doses ingested up to 45 g dietary protein(Reference Churchward-Venne, Pinckaers and Smeets6,Reference Holwerda, Paulussen and Overkamp25–Reference Kouw, Holwerda and Trommelen27) . In contrast, maximal stimulation of fractional muscle protein synthesis rates seems to occur at an ingested dose of about 20–30 g dietary protein(Reference Churchward-Venne, Pinckaers and Smeets6,Reference Holwerda, Paulussen and Overkamp25,Reference Moore, Robinson and Fry59,Reference Witard, Jackman and Breen60) . Ingestion of greater dosages of intrinsically labelled protein further increases dietary protein-derived amino acid incorporation into muscle tissue protein without a concomitant increase muscle protein synthesis rate(Reference Trommelen, Weijzen and van Kranenburg21,Reference Kouw, Holwerda and Trommelen27,Reference Trommelen, Kouw and Holwerda51) . This is the direct result of a greater post-prandial release of labelled amino acids and, as such, greater post-prandial precursor availability derived from the ingested protein source. Although resistance-type exercise is a strong anabolic stimulus that increases muscle protein synthesis rates and the incorporation of dietary protein-derived amino acids into muscle tissue, it does not result in a detectable increase in whole-body protein synthesis or net balance(Reference Trommelen, Holwerda and Kouw42,Reference Holwerda, Kouw and Trommelen43) . Taken together, these inconsistencies between various aspects of post-prandial protein handling teach us that we should be cautious when attempting to extrapolate between different metabolic outcomes.
Conclusions
Post-prandial protein handling in vivo in human subjects can be assessed by combining stable isotope-labelled amino acid infusions with the ingestion of intrinsically labelled protein with frequent collection of blood and muscle tissue samples. The combined ingestion of intrinsically labelled protein with intravenous stable isotope amino acid infusions allows for the simultaneous assessment of protein digestion and amino acid absorption kinetics (total amino acid rate of appearance, exogenous protein-derived amino acid rate of appearance, endogenous amino acid rate of appearance and total amino acid rate of disappearance), whole-body protein metabolism (whole-body protein synthesis, breakdown and oxidation rates and net protein balance) and skeletal muscle metabolism (muscle protein fractional synthesis rates and dietary protein-derived amino acid incorporation into muscle protein). Matching the labelled amino acid in intrinsically labelled protein with the intravenously infused labelled amino acid allows for a better maintenance of tracer steady-state conditions of the precursor pool and allows for a more accurate quantification of post-prandial muscle protein synthesis rates. The application of intrinsically labelled protein and intravenous stable isotope-labelled amino acid infusions has revealed that dietary protein-derived plasma amino acid availability can be strongly modulated by numerous nutritional and non-nutritional factors. This is of important clinical relevance, since dietary protein-derived amino acid availability is the main determinant driving the post-prandial increase in whole-body and skeletal muscle protein synthesis rates. The combination of ingesting intrinsically labelled protein with the continuous infusion of labelled amino acids is now frequently being applied to investigate how muscle protein synthesis rates are modulated by the various aspects of post-prandial protein handling and metabolism. It is therefore evident that the use of multiple stable isotope amino acid techniques, including intrinsically labelled proteins, will be critical in our efforts to understand various nutritional and non-nutritional factors impact post-prandial protein handling.
Financial Support
J. T. and L. v. L. have received research grants, consulting fees, speaking honoraria or a combination of these for work on post-prandial protein metabolism; full overview is provided at: https://www.maastrichtuni versity.nl/jorn.trommelen and https://www.maastrichtuniversity.nl/l.vanloon.
Conflict of Interest
None.
Authorship
J. T., A. M. H., P. J. M. P. and L. v. L. wrote the manuscript. All authors edited and approved the final version of the manuscript and agree to be accountable for all aspects of the work. Figures were created with Biorender.com.