- HLA
human leucocyte antigen
- IFN
interferon
- LT
leukotriene
- NSAID
non-steroidal anti-inflammatory drug
- RA
rheumatoid arthritis
Immune system overview
The immune system is responsible for the host's response to the presence of bacteria, viruses, fungi and parasites; it is also involved in protection against growth of certain tumours and in the response to injury and trauma. The immune system acts to distinguish between ‘self’ and ‘non-self’, permitting tolerance to self antigens and to non-threatening environmental agents such as food proteins and commensal gut bacteria. The system has two functional divisions: the innate (or natural) immune system and the acquired (also termed specific or adaptive) immune system. Both components involve various blood-borne factors and cells. Immune cells originate in bone marrow and are found circulating in the bloodstream, organised into lymphoid organs such as the thymus, spleen, lymph nodes and gut-associated lymphoid tissue or dispersed in other locations. The immune response is typified by cellular interactions and by the movement of cells to sites of infection or other immune activity. The four key functional activities of the immune response are:
to act as an exclusion barrier;
to distinguish self from non-self;
to develop tolerance to, or to eliminate the source of, non-self antigens;
to retain memory of immunological encounters.
In order to allow effective functioning of the immune system many different cell types, each specialised in a limited range of functions, are involved. These cells work in a coordinated integrated manner in order to assure a successful immune response.
Loss of tolerance can lead to disease and to adverse patient outcome. For example, autoimmune diseases result from loss of tolerance to self antigens, allergic diseases result from loss of tolerance to normally benign environmental or food components and inflammatory bowel diseases result from loss of tolerance to commensal gut bacteria. The loss of tolerance leads to an immunological response that is damaging to the host.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease that affects about 1% of the adult population and is more common in women than in men(Reference Firestein1). RA is characterised by symmetric polyarthritis(Reference Firestein1). Joint inflammation is manifested by swelling, pain, functional impairment, morning stiffness, osteoporosis and muscle wasting. Erosion of bone occurs commonly in the joints of the hands and feet. The joint lesions are characterised by infiltration of activated T lymphocytes, macrophages and antibody-secreting B lymphocytes into the synovium (the tissue lining the joints) and by proliferation of fibroblast-like synovial cells called synoviocytes(Reference Firestein1, Reference Sweeney and Firestein2). These cells and new blood vessels form a tissue termed pannus that leads to progressive destruction of cartilage and bone, which is most likely to be a result of cytokine- and eicosanoid-mediated induction of destructive enzymes such as matrix metalloproteinases. RA is also characterised by signs of systemic inflammation, such as elevated plasma concentrations of some cytokines (e.g. IL-6), acute-phase proteins and rheumatoid factors.
Genetic studies have linked susceptibility to, and severity of, RA to genes in the MHC II locus; in human subjects these genes encode the human leucocyte antigen (HLA) II proteins involved in antigen presentation. RA is associated with specific alleles of the HLA-DRB1 gene, although other HLA-DR alleles may also play a role(Reference Bowes and Barton3). As the function of HLA-DR is antigen presentation to T lymphocytes, the genetic association indicates a role for T-cells in RA(Reference Panayi4). In total the HLA region contributes 30–50% of the genetic component of RA. The second largest genetic risk for RA lies with a variant in the protein tyrosine phosphatase non-receptor 22 gene, which encodes an intracellular protein tyrosine phosphatase(Reference Bowes and Barton3). The variant may act to reduce the ability to down regulate activated T-cells. Recently, novel risk loci have been described(Reference Bowes and Barton3).
Synovial fluid from patients with RA contains high levels of pro-inflammatory cytokines including TNFα, IL-1β, IL-6, IL-8 and granulocyte–macrophage colony-stimulating factor(Reference Feldmann and Maini5). Synovial cells cultured ex vivo spontaneously produce TNFα, IL-1β, IL-6, IL-8 and granulocyte–macrophage colony-stimulating factor for extended periods of time(Reference Feldmann and Maini5). Synovial fluid from patients with RA also contains high levels of anti-inflammatory cytokines such as transforming growth factor β, IL-10, IL-1 receptor antagonist and soluble TNF receptors(Reference Feldmann and Maini5). Thus, the inflamed synovial joint contains excessive amounts of both pro- and anti-inflammatory cytokines, but given the ongoing state of inflammation there must be an imbalance in favour of the former.
Arachidonic acid, eicosanoids and the link with inflammation
Eicosanoids are key mediators and regulators of inflammation(Reference Lewis, Austen and Soberman6, Reference Tilley, Coffman and Koller7) and are generated from C20 PUFA. As inflammatory cells typically contain a high proportion of the n-6 PUFA arachidonic acid (20:4n-6) and low proportions of other C20 PUFA, arachidonic acid is usually the major substrate for eicosanoid synthesis. Eicosanoids, which include PG, thromboxanes, leukotrienes (LT) and other oxidised derivatives, are generated from arachidonic acid by the metabolic processes summarised in Fig. 1. They are involved in modulating the intensity and duration of inflammatory responses(Reference Lewis, Austen and Soberman6, Reference Tilley, Coffman and Koller7), have cell- and stimulus-specific sources and frequently have opposing effects. Expression of both isoforms of cyclooxygenase is increased in the synovium of patients with RA(Reference Feldmann and Maini5, Reference Sano, Hla, Maier, Crofford, Case, Maciag and Wilder8) and in joint tissues in rat models of arthritis(Reference Sano, Hla, Maier, Crofford, Case, Maciag and Wilder8). PGE2, LTB4 and 5-hydroxyeicosatetraenoic acid are found in the synovial fluid of patients with active RA(Reference Sperling9). Infiltrating leucocytes such as neutrophils, monocytes and synoviocytes are important sources of eicosanoids in RA(Reference Sperling9). PGE2 has a number of pro-inflammatory effects, including increasing vascular permeability, vasodilation, blood flow and local pyrexia and potentiation of pain caused by other agents. It also promotes the production of some matrix metalloproteinases and stimulates bone resorption. The efficacy of non-steroidal anti-inflammatory drugs (NSAID), which act to inhibit cyclooxygenase activity, in RA indicates the importance of this pathway in the pathophysiology of the disease. However, although these drugs provide rapid relief of pain and stiffness by inhibiting joint inflammation, they do not influence the course of the disease. LTB4 increases vascular permeability, enhances local blood flow, is a potent chemotactic agent for leucocytes, induces release of lysosomal enzymes and enhances release of reactive oxygen species and inflammatory cytokines such as TNFα, IL-1β and IL-6.
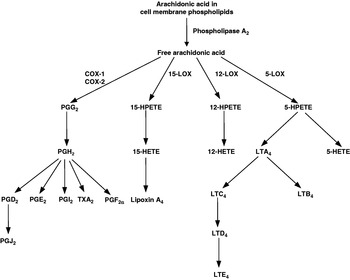
Fig. 1. Outline of the pathway of eicosanoid synthesis from arachidonic acid. COX, cyclooxygenase; HETE, hydroxyeicosatetraenoic acid; HPETE, hydroperoxyeicosatetraenoic acid; LOX, lipoxygenase; LT, leukotriene; TX, thromboxane.
Very-long-chain n-3 PUFA and inflammatory processes
Oily fish and fish oils contain the very-long-chain n-3 PUFA EPA (20:5n-3) and DHA (22:6n-3). Increased consumption of these fatty acids results in their incorporation into immune cell phospholipids(Reference Calder and Kremer10–Reference Calder13), which occurs in a dose–response fashion and is partly at the expense of arachidonic acid. The changed membrane fatty acid composition is believed to influence immune cell function and inflammatory processes(Reference Calder14) (Fig. 2). There have been numerous reviews of the influence of n-3 PUFA on many aspects of immune function in recent years(Reference Calder and Kremer10–Reference Switzer, McMurray and Chapkin27) and the reader is referred to these articles for details beyond those provided in the following sections.
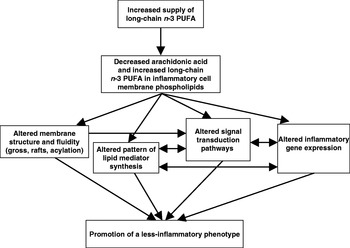
Fig. 2. Mechanisms by which n-3 PUFA can affect inflammatory cell activity.
Antigen-presenting cell function
There have been several studies of the effects of n-3 PUFA on MHC II or HLA expression or antigen presentation via class II molecules(Reference Calder28). These studies have typically found that class II expression and antigen presentation via class II molecules are decreased by n-3 PUFA. An in vitro study in which spleen cells were incubated with EPA has reported decreased ability of those cells to present antigen(Reference Fujikawa, Yamashita, Yamazaki, Sugiyama, Suzuki and Hamazaki29); this study did not report class II expression. Incubating murine macrophages with DHA decreases expression of the class II molecules (termed Ia in mice)(Reference Khair-el-Din, Sicher, Vazquez and Lu30). Likewise, incubating mouse macrophages with EPA or DHA decreases interferon (IFN)-γ-induced up-regulation of class II molecules(Reference Khair-el-Din, Sicher, Vazquez, Wright and Lu31) and incubating mouse dendritic cells with DHA decreases endotoxin-induced class II molecule up-regulation(Reference Weatherill, Lee, Zhao, Lemay, Youn and Hwang32) EPA and DHA treatment has been reported to diminish the up-regulation of HLA-DR and HLA-DP associated with IFN-γ stimulation of human monocytes(Reference Hughes, Southon and Pinder33). It has subsequently been demonstrated that these fatty acids decrease the ability of human monocytes to present antigen(Reference Hughes and Pinder34). Three studies, one in mice(Reference Huang, Misfeldt and Fritsche35), one in rats(Reference Sanderson, MacPherson, Jenkins and Calder36) and one in human subjects(Reference Hughes, Pinder, Piper, Johnson and Lund37) have reported effects of dietary n-3 PUFA on class II expression. Feeding mice fish oil, which contains EPA and DHA, results in a reduction in MHC II expression on peritoneal cells (mainly B lymphocytes and macrophages)(Reference Huang, Misfeldt and Fritsche35). A human supplementation study with fish oil has reported decreased expression of HLA-DR, -DP and -DQ on IFN-γ-stimulated blood monocytes(Reference Hughes, Pinder, Piper, Johnson and Lund37), with similar effects to those seen with n-3 PUFA in vitro (Reference Hughes, Southon and Pinder33). These studies did not examine antigen presentation activity. However, a study that involved feeding an EPA-rich oil to mice has shown decreased antigen (keyhole limpet (Megathura crenulata) haemocyanin) presentation by spleen cells to T-cell clones(Reference Fujikawa, Yamashita, Yamazaki, Sugiyama, Suzuki and Hamazaki29). Perhaps the most thorough study of this type to date is that in which feeding a fish oil-rich diet to rats was found to result in decreased expression of MHC II on dendritic cells(Reference Sanderson, MacPherson, Jenkins and Calder36). These cells were found to have a much reduced capacity to present antigen (keyhole limpet haemocyanin) to antigen-sensitised spleen T-cells. The reduction in antigen presentation is probably much greater than could be explained by the reduction in class II expression, suggesting that other interactions between antigen-presenting cells and T lymphocytes are affected by dietary n-3 PUFA. It was reported that levels of the co-stimulatory molecules CD2, CD11a and CD18 are also decreased on dendritic cells from fish oil-fed rats(Reference Sanderson, MacPherson, Jenkins and Calder36).
T lymphocyte reactivity
In vitro studies have demonstrated that EPA and DHA decrease T-cell proliferation(Reference Calder and Newsholme38–Reference Calder, Yaqoob, Harvey, Watts and Newsholme41) and the production of helper T-cell 1-type cytokines such as IL-2(Reference Calder and Newsholme38, Reference Calder and Newsholme39, Reference Wallace, Miles, Evans, Stock, Yaqoob and Calder42). Feeding studies in rodents and supplementation studies in human subjects have also shown that fish oil decreases T-cell proliferation(Reference Yaqoob, Newsholme and Calder43–Reference Trebble, Wootton, Miles, Mullee, Arden, Ballinger, Stroud and Calder48) and production of helper T-cell 1-type cytokines such as IL-2(Reference Wallace, Miles, Evans, Stock, Yaqoob and Calder42, Reference Jolly, Jiang, Chapkin and McMurray45, Reference Meydani, Endres, Woods, Goldin, Soo, Morrill-Labrode, Dinarello and Gorbach47, Reference Trebble, Wootton, Miles, Mullee, Arden, Ballinger, Stroud and Calder48) and IFN-γ(Reference Wallace, Miles, Evans, Stock, Yaqoob and Calder42, Reference Trebble, Wootton, Miles, Mullee, Arden, Ballinger, Stroud and Calder48), although it is important to note that not all human studies report such an effect(Reference Calder11). The reason for these discrepancies in the literature is not entirely clear, but dose of n-3 PUFA used, technical factors and differences among subjects studied are likely to be contributing factors.
The mechanism by which long-chain n-3 PUFA affect T-cell reactivity was initially thought to relate to altered patterns of eicosanoid synthesis; however, through the use of eicosanoid synthesis inhibitors and pure eicosanoids in vitro this mechanism has been shown to be unlikely(Reference Calder, Bevan and Newsholme40). Studies over the last few years have demonstrated that the inhibitory effects of n-3 PUFA in general, and of EPA in particular, relate to membrane-mediated effects that impact on the early stages of cell signalling(Reference Sanderson and Calder49–Reference Zeyda, Szekeres, Säemann, Geyeregger, Stockinger, Zlabinger, Waldhäusl and Stulnig52).
Inflammatory mediator production
Eicosanoids. Increased consumption of very-long-chain n-3 PUFA such as EPA and DHA, results in decreased amounts of arachidonic acid present in immune cell membranes and available for synthesis of eicosanoids(Reference Calder and Kremer10–Reference Calder13). Thus, feeding fish oil to laboratory rodents or supplementing the diet of human subjects with fish oil has been reported to result in decreased production of a range of eicosanoids including PGE2, thromboxane B2, LTB4, 5-hydroxyeicosatetraenoic acid and LTE4 by inflammatory cells(Reference Calder and Kremer10–Reference Calder13). A recent study has demonstrated the dose–response effect to dietary EPA of PGE2 production by endotoxin-stimulated human mononuclear cells and suggests that an EPA intake of >2 g/d is required in order to be effective(Reference Rees, Miles, Banerjee, Wells, Roynette, Wahle and Calder53).
EPA is also able to act as a substrate for both cyclooxygenase and 5-lipoxygenase, giving rise to eicosanoids with a slightly different structure from those formed from arachidonic acid. Thus, fish oil supplementation of the human diet has been shown to result in increased production of LTB5, LTE5 and 5-hydroxyeicosapentaenoic acid by inflammatory cells(Reference Calder and Kremer10–Reference Calder13). The functional importance of this outcome is that the mediators formed from EPA are frequently less potent than those formed from arachidonic acid; for example, LTB5 is less potent as a neutrophil chemotactic agent than LTB4(Reference Goldman, Pickett and Goetzl54, Reference Lee, Mencia-Huerta, Shih, Corey, Lewis and Austen55).
Resolvins and related compounds: novel EPA- and DHA-derived anti-inflammatory mediators
Recent studies have identified a novel group of trihydroxyeicosapentaenoic acid mediators, termed E-series resolvins, formed from EPA by a series of reactions involving cyclooxygenase-2 (acting in the presence of aspirin) and 5-lipoxygenase. These mediators appear to exert potent anti-inflammatory actions(Reference Serhan, Clish, Brannon, Colgan, Gronert and Chiang56–Reference Serhan, Hong, Gronert, Colgan, Devchand, Mirick and Moussignac58). In addition, DHA-derived trihydroxydocosahexanoic acid mediators termed D-series resolvins are produced by a similar series of reactions and these resolvins are also anti-inflammatory(Reference Hong, Gronert, Devchand, Moussignac and Serhan59, Reference Marcheselli, Hong, Lukiw and Hua60). Metabolism of DHA via a series of steps, several involving 5-lipoxygenase, generates a dihydroxydocosatriene termed neuroprotectin D1, again a potent anti-inflammatory molecule(Reference Mukherjee, Marcheselli, Serhan and Bazan61). The identification of these novel EPA- and DHA-derived mediators is an exciting new area of n-3 fatty acids and inflammatory mediators and the implications to a variety of conditions may be of great importance(Reference Serhan, Arita, Hong and Gotlinger62, Reference Serhan63).
Inflammatory cytokines
Cell-culture studies have demonstrated that EPA and DHA can inhibit the production of IL-1β and TNFα by monocytes(Reference Babcock, Novak, Ong, Jho, Helton and Espat64) and the production of IL-6 and IL-8 by venous endothelial cells(Reference De Caterina, Cybulsky, Clinton, Gimbrone and Libby65, Reference Khalfoun, Thibault, Watier, Bardos and Lebranchu66). Fish oil feeding decreases ex vivo production of TNFα, IL-1β and IL-6 by rodent macrophages(Reference Billiar, Bankey, Svingen, Curran, West, Holman, Simmons and Cerra67–Reference Yaqoob and Calder69). Supplementation of the diet of healthy human volunteers with fish oil decreases production of TNF or IL-1 or IL-6 by mononuclear cells in some studies(Reference Calder and Kremer10–Reference Calder13), although a number of other studies have shown little effect of n-3 PUFA on production of inflammatory cytokines in human subjects(Reference Calder11). The reason for these discrepancies in the literature is not entirely clear, but dose of n-3 PUFA used, technical factors and differences among subjects studied, including genetic differences(Reference Grimble, Howell, O'Reilly, Turner, Markovic, Hirrell, East and Calder70, Reference Shen, Arnett and Peacock71), are likely to be contributing factors.
n-3 PUFA and animal models of rheumatoid arthritis
The effects of n-3 PUFA from fish oil on antigen presentation, T-cell reactivity and inflammatory lipid and peptide mediator production (Fig. 3) suggest that these fatty acids might have a role both in decreasing the risk of development of RA and in decreasing severity in those patients with the disease. Indeed, dietary fish oil has been shown to have beneficial effects in animal models of arthritis. For example, compared with vegetable oil, feeding mice fish oil delays the onset (mean 34 d v. 25 d) and reduces the incidence (69% v. 93%) and severity (mean peak severity score 6·7 v. 9·8) of type II collagen-induced arthritis(Reference Leslie, Gonnerman, Ullman, Hayes, Franzblau and Cathcart72). In another study both EPA and DHA were found to suppress streptococcal cell wall-induced arthritis in rats, with EPA being more effective(Reference Volker, FitzGerald and Garg73).
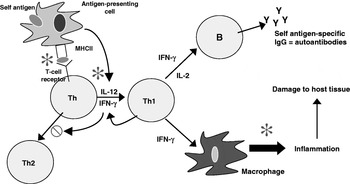
Fig. 3. Cellular sites of anti-inflammatory actions of long-chain n-3 PUFA. IFN, interferon; Th, helper T-cell; Y, IgG. ![]() , Sites of action of n-3 PUFA;
, Sites of action of n-3 PUFA; ![]() , inhibits.
, inhibits.
Trials of n-3 PUFA in rheumatoid arthritis
Several studies have reported anti-inflammatory effects of fish oil in patients with RA, such as decreased LTB4 production by neutrophils(Reference Kremer, Bigauoette, Michalek, Timchalk, Lininger, Rynes, Huyck, Zieminski and Bartholomew74–Reference van der Tempel, Tullekan, Limburg, Muskiet and van Rijswijk77) and monocytes(Reference Cleland, French, Betts, Murphy and Elliot76, Reference Tullekan, Limburg, Muskiet and van Rijswijk78), decreased PGE2 production by mononuclear cells(Reference Cleland, Caughey, James and Proudman79), decreased IL-1 production by monocytes(Reference Kremer, Lawrence, Jubiz, DiGiacomo, Rynes, Bartholomew and Sherman80), decreased plasma IL-1β concentrations(Reference Esperson, Grunnet, Lervang, Nielsen, Thomsen, Faarvang, Dyerberg and Ernst81), decreased serum C-reactive protein concentrations(Reference Kremer, Bigauoette, Michalek, Timchalk, Lininger, Rynes, Huyck, Zieminski and Bartholomew74, Reference Sundrarjun, Komindr, Archararit, Dahaln, Puchaiwatananon, Angthararak, Udomsuppayakui and Chuncharunee82) and normalisation of the neutrophil chemotactic response(Reference Sperling, Weinblatt, Robin, Ravalese, Hoover, House, Coblyn, Fraser, Spur and Robinson83). A number of randomised placebo-controlled double-blind studies of fish oil in RA have been reported. The characteristics and findings of these trials are summarised in Table 1. The dose of long-chain n-3 PUFA used in these trials was between 1·6 and 7·1 g/d and averaged about 3·5 g/d (see Table 1). Almost all these trials have shown some benefit of fish oil (Table 1). Such benefits include reduced duration of morning stiffness, reduced number of tender or swollen joints, reduced joint pain, reduced time to fatigue, increased grip strength and decreased use of non-steroidal anti-inflammatory drugs (Table 1). A number of reviews of these trials have been published(Reference James and Cleland84–Reference Cleland, James and Proudman90) and each has concluded that there is benefit from fish oil. In an editorial commentary discussing the use of fish oil in RA it was concluded that ‘the findings of benefit from fish oil in rheumatoid arthritis are robust’, ‘dietary fish oil supplements in rheumatoid arthritis have treatment efficacy’ and ‘dietary fish oil supplements should now be regarded as part of the standard therapy for rheumatoid arthritis’(Reference Cleland and James91). A meta-analysis that included data from nine trials published between 1985 and 1992 inclusive and from one unpublished trial has concluded that ‘dietary fish oil supplementation for three months significantly reduced tender joint count (mean difference −2·9; P=0·001) and morning stiffness (mean difference –25·9 min; P=0·01)’(Reference Fortin, Lew, Liang, Wright, Beckett, Chalmers and Sperling92). A more recent meta-analysis of data from trials published between 1985 and 2002 included one study of flaxseed oil, one study that did not use a control for fish oil and one study in which transdermal administration of n-3 PUFA by ultrasound, rather than the oral route, was used(Reference MacLean, Mojica and Morton93). This meta-analysis has concluded that fish oil supplementation has no effect on ‘patient report of pain, swollen joint count, disease activity or patient's global assessment’. However, this conclusion may be flawed, because of the inappropriate manner in which studies were combined and because of a poor understanding of the study designs used. For example, the meta-analysis fails to recognise that patients' ability to reduce the need for using NSAID or their ability to be withdrawn from NSAID, as was done in some designs, must indicate a reduction in pain with n-3 PUFA use. This meta-analysis does state that ‘in a qualitative analysis of seven studies that assessed the effect of n-3 fatty acids on anti-inflammatory drug or corticosteroid requirement, six demonstrated reduced requirement for these drugs’ and concludes that ‘n-3 fatty acids may reduce requirements for corticosteroids’(Reference MacLean, Mojica and Morton93). The effects of long-chain n-3 PUFA on tender joint count were not assessed by this meta-analysis, which reiterated the findings of the earlier meta-analysis that ‘n-3 fatty acids reduce tender joint counts’(Reference Fortin, Lew, Liang, Wright, Beckett, Chalmers and Sperling92). A recent meta-analysis of n-3 PUFA with data from seventeen trials included one trial of RA with flaxseed oil and two trials of fish oil not in patients with RA, but which reported joint pain(Reference Goldberg and Katz94). Data on six outcomes were analysed and are summarised in Table 2. This meta-analysis provides further evidence of the robustness of the efficacy of n-3 PUFA in RA.
Table 1. Summary of the results of placebo-controlled studies using dietary long-chain n-3 PUFA (in the form of fish oil) in patients with rheumatoid arthritis
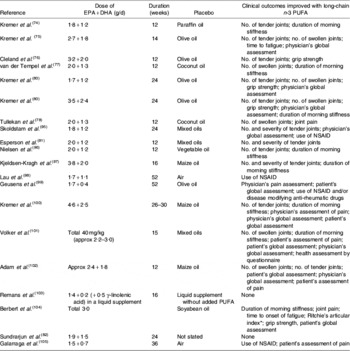
Approx, approximately; NSAID, non-steroidal anti-inflammatory drugs.
* Based on the summation of a number of quantitative evaluations of the pain experienced by the patient when the joints were subjected to pressures when exerted over the articular margin or in some instances on movement of the joint(Reference Ritchie, Boyle, McInnes, Jasani, Dalakos, Grieveson and Buchanan106).
Table 2. Summary of the findings of the meta-analysis of Goldberg & Katz(Reference Goldberg and Katz94)
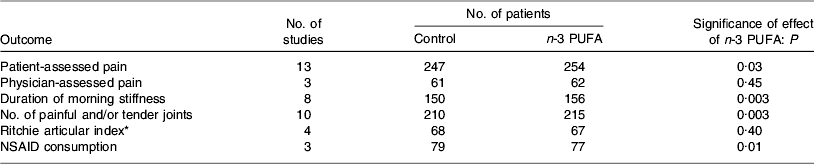
NSAID, non-steroidal anti-inflammtory drugs.
* Based on the summation of a number of quantitative evaluations of the pain experienced by the patient when the joints were subjected to pressures when exerted over the articular margin or in some instances on movement of the joint(Reference Ritchie, Boyle, McInnes, Jasani, Dalakos, Grieveson and Buchanan106).
Several other studies have also provided information about the benefits of n-3 PUFA in RA. For example, in a study that has compared outcomes among patients with RA who did and did not consume fish oil supplements it was found that fish oil users are more likely to reduce use of NSAID and are more likely to be in remission(Reference Cleland, Caughey, James and Proudman79).
Overall conclusions
Eicosanoids derived from the n-6 PUFA arachidonic acid play a role in RA, and the efficacy of NSAID in RA indicates the importance of pro-inflammatory cyclooxygenase pathway products in the pathophysiology of the disease. At sufficiently high intakes long-chain n-3 PUFA decrease the production of inflammatory eicosanoids from arachidonic acid and promote the production of less-inflammatory eicosanoids from EPA and of anti-inflammatory resolvins and similar mediators from EPA and DHA. Long-chain n-3 PUFA have other anti-inflammatory actions including decreasing antigen presentation via MHC II, decreasing T-cell reactivity and helper T-cell 1-type cytokine production and decreasing inflammatory cytokine production by monocytes and macrophages. Work with animal models of RA has demonstrated the efficacy of fish oil. There have been a number of clinical trials of fish oil in patients with RA. Most of these trials have reported clinical improvements (e.g. improved patient assessed pain, decreased morning stiffness, fewer painful or tender joints, decreased use of NSAID), and when the trials have been pooled in meta-analyses significant clinical benefit has emerged.
Acknowledgements
The author is a consultant to Vifor Pharma, Villars-sur-Glane, Switzerland and Danone Research Centre for Specialised Nutrition, Wageningen, The Netherlands.







