

1. Preamble
Guidelines summarize and evaluate all available evidence with the aim of assisting physicians in selecting the best management strategy for an individual patient suffering from a given condition, taking into account the impact on outcome and the risk–benefit ratio of diagnostic or therapeutic means. Guidelines are no substitutes for textbooks, primary literature sources, or clinical evaluation and judgment. Guidelines and recommendations should help physicians to make decisions in their daily practice. However, the ultimate specific decisions regarding the care of an individual patient must be made by his/her responsible physician(s).
A large number of guidelines have been issued in recent years by many societies and organizations. Because of the impact of guidelines on clinical practice, quality criteria for their development have been established to make the formulation of guidelines transparent to the user.
We followed the recommendations for formulating guidelines issued by the European Association for Cardio-Thoracic Surgery (EACTS) [Reference Sousa-Uva, Head, Thielmann, Cardillo, Benedetto and Czerny1].
Members of this Committee were selected by the EACTS Congenital Domain and the Association for European Paediatric and Congenital Cardiology (AEPC) to represent all specialities involved with the medical and surgical care of patients with transposition of the great arteries (TGA). The following complex transposition cases remain outside the scope of this article: TGA with aortic coarctation or arch hypoplasia, TGA with ventricular septal defect (VSD) with or without left ventricular outflow tract (LVOT) obstruction, TGA or malposition of the great arteries associated with double-outlet right ventricle or in anatomical or functionally univentricular hearts, and congenitally corrected transposition. In brief, experts in the field were selected and undertook a comprehensive review of the published evidence for management of the various clinically important aspects of this common congenital cardiac anomaly. A critical evaluation of diagnostic and therapeutic procedures was performed. The level of evidence and the strength of recommendation of particular management options were weighed and graded according to predefined scales, as depicted in Tables 1 and 2.
Table 1 Classes of recommendations

Table 2 Levels of evidence

A uniform wording of the stated recommendations to reflect the strength of evidence has been used, in accordance with EACTS policy, as recently published [Reference Sousa-Uva, Head, Thielmann, Cardillo, Benedetto and Czerny1].
Disclosure: The members of the Task Force have provided disclosure statements of all relationships that might be perceived as real or potential sources of conflicts of interest. These disclosure forms are kept on file at the headquarters of the EACTS/AEPC. The Committee report received its entire financial support from the EACTS and AEPC, without any involvement of the pharmaceutical, device or surgical industries.
The Task Force selected by the EACTS and AEPC is responsible for the endorsement process of these joint guidelines. The finalized document has been approved by all the experts involved in the Committee.
The document was revised, and finally approved, by both the EACTS and the AEPC and subsequently submitted for publication simultaneously to the European Journal of Cardio-Thoracic Surgery and Cardiology in the Young.
Limitations: Practice guidelines are to be evidence based, but, in the field of congenital heart disease, most studies involve relatively small patient numbers for any given condition, especially when variants and coexisting lesions are considered. Therefore, there is a paucity of robust data such as prospective randomized trials; consequently, it is frequently impossible to use categories for strength of endorsement that have been used in guidelines documents pertaining to other disciplines. Thus, the vast majority of recommendations in this document are based on expert consensus (level of evidence C) rather than on solid data (level of evidence A or B).
2. Background
Transposition of the great arteries is the most common cyanotic congenital heart defect [Reference Marek, Tomek, Skovranek, Povysilova and Samanek2]. It accounts for approximately 5% of congenital heart disease cases and is characterized by ventriculo-arterial discordance: the left ventricle gives rise to the pulmonary artery and the right ventricle, to the aorta. There is atrioventricular concordance. If no significant additional cardiac lesions are present, it is referred to as TGA with intact ventricular septum (TGA IVS). The lesion is categorized as complex TGA when it has associated cardiac anomalies including VSD (which occurs in up to 45% of cases), LVOT obstruction (25%) and coarctation of the aorta (5%). In general, TGA is not familial. There is no known association with syndromes or chromosomal abnormalities. There is a 2:1 male preponderance.
The anatomical configuration of this anomaly establishes a potentially fatal parallel circulation that results in deep hypoxaemia from lack of mixing, with resulting lactic acidosis and demise. Prompt, adequate preoperative intervention and stabilization, followed by surgical repair and expert postoperative management, favour an excellent outcome, with short-term survival probability around 97–100% in selected centres [Reference Fricke, D’Udekem, Richardson, Thuys, Dronavalli and Ramsay3–Reference Stoica, Carpenter, Campbell, Mitchell, da Cruz and Ivy7]. The arterial switch operation (ASO), first described by Adib Jatene in 1976 [Reference Jatene, Fontes, Paulista, Souza, Neger and Galantier8], is currently the procedure of choice when the anatomical conditions and the timeline are appropriate; it is performed in the first month of life. Other alternatives, such as the atrial switch and the two-stage ASO, are reserved for the specific scenarios discussed below. Despite the medical and surgical advances in the management of TGA and the low mortality rate, patients require expert diagnostic evaluation, preferably prenatally, and meticulous multidisciplinary management in the perinatal period, preoperatively, intraoperatively and postoperatively.
3. Diagnosis
3.1 Prenatal diagnosis
3.1.1 Prenatal detection
The diagnosis of TGA can be made accurately before birth if the foetal heart is screened at the time of the obstetric anomaly scan. Some studies have shown that the type of repair likely to be required after birth can be well predicted [Reference Bonnet, Coltri, Butera, Fermont, Le Bidois and Kachaner9–Reference Sivanandam, Glickstein, Printz, Allan, Altmann and Solowiejczyk12]. Due to the fact that the previously frequently used four-chamber view in TGA IVS shows no abnormality, the overall proportion of cases of TGA in foetal series has been low compared with postnatal series [Reference Allan, Sharland, Milburn, Lockhart, Groves and Anderson13–Reference Jaeggi, Sholler, Jones and Cooper15]. The inclusion of the outflow-tract views at the time of the obstetric foetal anomaly scan results in significant improvement in prenatal detection of the transposition [Reference Sharland16,Reference Wigton, Sabbagha, Tamura, Cohen, Minogue and Strasburger17]. Recent publications have reported improved prenatal detection rates for TGA of up to 50% [Reference Marek, Tomek, Skovranek, Povysilova and Samanek2, Reference Khoshnood, Lelong, Houyel, Thieulin, Jouannic and Magnier18–Reference van Velzen, Haak, Reijnders, Rijlaarsdam, Bax and Pajkrt20]. It is now generally more widely recommended that, in addition to the four-chamber view, the views of the cardiac outflow tracts also be included as part of the obstetric screening scan [21–23]. A formal programme for education and training regarding the foetal heart is required as part of this process, to ensure that sonographers are taught and can maintain the skills of foetal heart examination [Reference Hunter, Heads, Wyllie and Robson24–Reference Tegnander and Eik-Nes28].
3.1.2 Counselling following prenatal detection
If transposition is detected or even suspected from the obstetric anomaly scan, referral should be made to a specialist who is experienced in the diagnosis and management of congenital heart disease in the foetus. This referral should be made as soon as possible after detection of a possible transposition, to have the diagnosis confirmed and to allow the parents to be counselled appropriately [29, Reference Allan, Dangel, Fesslova, Marek, Mellander and Oberhansli30].
Following the diagnosis of TGA, the parents need to be informed of the diagnosis, associations, further management during pregnancy and birth, management after birth and the prognosis for their baby. They also need to be made aware of features that may complicate the management. The parents should be given all the information regarding their baby’s heart condition in a way that they understand and be allowed sufficient time to ask questions. Written information and drawings illustrating the problem should be provided. The parents should be given the opportunity to speak with a paediatric cardiac surgeon as well as having the option to speak with other parents who have had a child with transposition. Contact details of parent support groups, both locally and nationally, can be provided to help them.
3.1.3 Further prenatal management
Because many forms of congenital heart disease are associated with extracardiac abnormalities, including karyotype abnormalities, foetal karyotyping is generally recommended after prenatal diagnosis [Reference Copel, Pilu and Kleinman31–Reference Tennstedt, Chaoui, Korner and Dietel34]. However, cases of TGA are rarely associated with chromosomal anomalies. It is important to liaise with foetal medicine specialists in order to exclude any associated extracardiac abnormalities. Foetal karyotyping is not generally indicated or recommended in TGA IVS, but the option of karyotyping can be discussed on an individual basis. Following the initial diagnosis and counselling, further foetal cardiology assessment will be required later in the pregnancy. The number and timing of further scans may vary depending on local practices, but they should include an assessment in the few weeks prior to delivery to look for high-risk features [Reference Jouannic, Gavard, Fermont, Le Bidois, Parat and Vouhé35–Reference Punn and Silverman37].

a Class of recommendation.
b Level of evidence.
Recommendations for prenatal detection
3.2 Perinatal management—timing, place and mode of delivery
Studies comparing the outcome of babies with TGA diagnosed prenatally with those diagnosed postnatally suggest that the rates of preoperative and postoperative mortality [Reference Bonnet, Coltri, Butera, Fermont, Le Bidois and Kachaner9, Reference van Velzen, Haak, Reijnders, Rijlaarsdam, Bax and Pajkrt20, Reference Fuchs, Muller, Abdul-Khaliq, Harder, Dudenhausen and Henrich38, Reference Khoshnood, De Vigan, Vodovar, Goujard, Lhomme and Bonnet39] and morbidity [Reference Escobar-Diaz, Freud, Bueno, Brown, Friedman and Schidlow19, Reference Bartlett, Wypij, Bellinger, Rappaport, Heffner and Jonas40–Reference Verheijen, Lisowski, Stoutenbeek, Hitchcock, Bennink and Meijboom43] are lower for babies diagnosed prenatally.
3.2.1 Site and timing of delivery
Because babies with TGA require early treatment after birth, it is generally recommended that delivery takes place at or near a tertiary-care paediatric cardiology and paediatric cardiac surgery centre [Reference Hellstrom-Westas, Hanseus, Jogi, Lundstrom and Svenningsen44, Reference Mirlesse, Cruz, Le Bidois, Diallo, Fermont and Kieffer45]. Adhering to this practice enables the neonate to be in optimal condition and avoids neonatal retrieval transport-related complications and costs [Reference Jegatheeswaran, Oliveira, Batsos, Moon-Grady, Silverman and Hornberger46]. Although the delivery must be scheduled before the due date, the majority of women can have a vaginal delivery, which is generally recommended [Reference Landis, Levey, Levasseur, Glickstein, Kleinman and Simpson47]. However, a planned caesarean delivery may be indicated if high-risk maternal or foetal features are identified.

a Class of recommendation.
b Level of evidence.
Recommendations for perinatal management
3.3 Postnatal diagnosis
3.3.1 Postnatal detection
The newborn with TGA and inadequate intercirculatory mixing will be symptomatic from birth. Severe cyanosis is an early, almost universal clinical finding, which at least during the first hours after birth, may be the only sign. Screening for arterial oxygen saturation (SaO2) is indicated for early identification of initially asymptomatic patients with TGA, when the pre- or post-ductal value or both are <95% [Reference Eckersley, Sadler, Parry, Finucane and Gentles48].
3.3.2 Further diagnostic steps
Once cyanotic congenital heart disease is suspected, transthoracic echocardiography should be performed immediately, because duration of deep cyanosis and tissue hypoxia are important additional factors in determining ventricular function, acidosis and eventually multiple organ failure.
The results observed on chest radiographs can be normal, but the following abnormal features can also be observed: oval or egg-on-side cardiac shape (due to the narrow mediastinum), mild cardiomegaly and increased pulmonary vascular markings. The electrocardiogram (ECG) may be normal, with the typical neonatal findings of right-axis deviation and right ventricular hypertrophy. Echocardiography is the modality of choice for a definitive diagnosis.
At the time of echocardiography, one should pay particular attention to the root of the great arteries and to the coronary arteries or concomitant features such as VSD, LVOT obstruction, coarctation and mitral valve anomalies. In particular, the diameters of the main pulmonary artery and the aorta have to be measured; the location of the valve commissures and also the origin and course of the coronary arteries must be described carefully before surgery.
It has been shown that echocardiography facilitates accurate evaluation of the coronary artery pattern and exclusion of other relevant malformations [Reference McMahon, el Said, Feltes, Watrin, Hess and Fraser49, Reference Pasquini, Sanders, Parness, Wernovsky, Mayer and Van der Velde50]. In addition, echocardiography facilitates imaging for the safe performance of balloon atrial septostomy (BAS) (also known as the Rashkind procedure). Because BAS can be safely performed under echocardiographic guidance, preoperative diagnostic cardiac catheterization should be considered only in selected cases for diagnosis of complex lesions or if institutional experience does not permit performance of atrial septostomy under echocardiographic guidance.
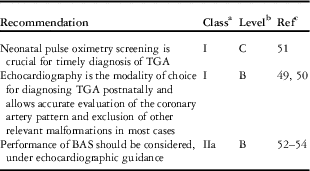
BAS: balloon atrial septostomy; TGA: transposition of the great arteries.
a Class of recommendation.
b Level of evidence.
c References
Recommendations for postnatal diagnosis
4. Perinatal management
Significant regional differences exist in the organization of care for newborns with TGA. In most instances, especially in the absence of prenatal diagnosis, the newborn with TGA will need to be stabilized in a neonatal intensive care unit (ICU) and subsequently transported to a tertiary-care centre, where definitive surgical care is available. Although elective intubation of infants on prostaglandin E1 (PGE1) prior to transport has been common practice in many institutions, several studies have shown that the rate of complications is significantly higher in infants who need intubation [Reference Browning Carmo, Barr, West, Hopper, White and Badawi55, Reference Meckler and Lowe56]. Occasionally, BAS may be available locally and may be performed prior to transport.
4.1 Monitoring and immediate care
Preoperative monitoring of patients with TGA in the ICU includes mostly noninvasive technologies associated with the clinical evaluation of vital signs and peripheral perfusion and cardiovascular examination (although invasive strategies may be required): pre- and post-ductal pulse oximetry, continuous ECG, noninvasive blood pressure monitoring, respiratory rate and pattern monitoring. Inspired end-tidal capnography may be used and reserved for ventilated patients. In addition to vital signs, urine output must be monitored closely, but the insertion of a Foley catheter is not justified unless the patient is haemodynamically compromised. Tissue perfusion monitoring may be followed by serial testing for blood lactate levels and near-infrared spectroscopy (NIRS) in the decompensated phase. Neonates with TGA might have umbilical venous, and eventually umbilical arterial, lines inserted promptly after birth, which facilitates safe administration of drugs, including—but not exclusively limited to—PGE1; surveillance of invasive haemodynamic parameters, as needed; fluid administration and acid–base follow-up and management. The use of other central lines should be minimized in the preoperative period unless the patient remains critically ill. To assess mixed venous saturations, sampling in the innominate vein is required to avoid overestimates because of the atrial level mixing. Fluids ought to be administered without restrictions, and the indications do not vary with standard neonatal recommendations.
4.2 Haemodynamic management
The initial management of newborns with TGA should focus on stabilization, optimization of mixing of systemic and pulmonary circulations (management of hypoxia) and oxygen delivery, maintenance of adequate systemic perfusion and correction of acidosis.
The immediate priority after birth and throughout the first few hours of life is to determine whether the mixing between systemic and pulmonary circulations is adequate. Immediately after birth, an intravenous (IV) infusion of PGE1 is recommended to maintain ductal patency until the comprehensive series of postnatal echocardiograms is complete and all sites of intercirculatory mixing have been evaluated. PGE1 has been used in various dosing regimens: a higher dose of up to 0.1 µg/kg/min may be necessary when the ductus needs to be reopened. To maintain patency, starting doses vary from 0.0125 to 0.05 µg/kg/min, and patients can be weaned, starting 2–4 h following initiation, provided that oxygen (O2) saturations and tissue perfusion remain acceptable. Nevertheless, the use of PGE1 may not suffice, because ductal shunting is often inadequate in the presence of a restrictive interatrial communication. These patients warrant an emergent atrial septostomy. Throughout the performance of the atrial septostomy, or in those patients needing longer-term ventilation, sedation and analgesia are occasionally required. The usual combination of drugs includes nonopioids (i.e. paracetamol), opioids (low-dose morphine or fentanyl) and benzodiazepines. Dexmedetomidine has emerged as a useful and safe drug with anxiolytic properties and no significant respiratory depressive effect [Reference Barton, Munoz, Morell and Chrysostomou57].
Patients presenting with deep hypoxaemia, acidosis and in shock must benefit from the emergent measures recommended in neonatal advanced life-support algorithms. Concomitantly, an infusion of PGE1 should be emergently started at high doses (0.1 µg/kg/min) while preparing for the atrial septostomy.
Once adequate mixing has been achieved at the atrial level, discontinuation of PGE1 is often possible, unless there is an associated left-heart obstruction (i.e. coarctation of the aorta). Notwithstanding this attempt, successful discontinuation of the drug is unpredictable [Reference Finan, Mak, Bismilla and McNamara58, Reference Oxenius, Hug, Dodge-Khatami, Cavigelli-Brunner, Bauersfeld and Balmer59]. Because of the risk of rebound hypoxaemia after abrupt discontinuation of PGE1, it is recommended that, after septostomy, patients should be weaned from PGE1 rather than stopped. Patients remaining on PGE1 must be observed for potential side and adverse effects. The risk of apnoea may be attenuated by the administration of caffeine or with stimulation tools like a high-flow nasal cannula or continuous positive airway pressure [Reference Lim, Kulik, Kim, Charpie, Crowley and Maher60]. Furthermore, persistent left-to-right shunting across the ductus arteriosus may cause pulmonary oedema, which may affect patient stability and require escalation of therapy and airway support. Pragmatically, it may be adequate to adopt a permissive attitude with regards to the degree of cyanosis rather than exposing patients to the deleterious effects of excessive blood flow to maintain a higher arterial O2 saturation level.
Further haemodynamic measures to support decompensated patients include colloids or crystalloids for volume expansion, use of O2 and correction of metabolic acidosis.
A few neonates may remain significantly cyanotic and acidotic even after the atrial septostomy. In such circumstances, echocardiography should be performed to confirm the unrestrictive nature of the atrial septal defect (ASD) as well as of the patent ductus arteriosus and to determine the presence and degree of pulmonary hypertension. The diagnosis of pulmonary hypertension is usually confirmed using echocardiography. Although cut-off values are difficult to define, the rate of diagnosed pulmonary hypertension varies in the available literature. The incidence of persistent pulmonary hypertension in neonates with TGA is 12.5%, and it occurs more frequently in cases of TGA IVS [Reference Roofthooft, Bergman, Waterbolk, Ebels, Bartelds and Berger61, Reference Newfeld, Paul, Muster and Idriss62].
Mortality is high in this group of neonates and mid-term postoperative outcomes are negatively affected [Reference Roofthooft, Bergman, Waterbolk, Ebels, Bartelds and Berger61, Reference Fan, Hu, Zheng, Li, Zhang and Pan63]. Given that it is a serious condition with a high mortality rate, different treatment strategies have been used with variable success, including sedation, paralysis and hyperventilation [Reference Chang, Wernovsky, Kulik, Jonas and Wessel64], inhaled nitric oxide (NO) [Reference El-Segaier, Hellstrom-Westas and Wettrell65], sildenafil, bosentan [Reference Goissen, Ghyselen, Tourneux, Krim, Storme and Bou66] and extracorporeal life support (ECLS), alone or in combination [Reference Jaillard, Belli, Rakza, Larrue, Magnenant and Rey67, Reference Luciani, Chang and Starnes68]. Because the existing literature consists mainly of case reports, management should include the stepwise introduction of the above-mentioned treatment modalities and close monitoring of the clinical response (improved oxygenation). Such patients may require the resumption of PGE1 because the ductus arteriosus may be useful as a ‘pop-off’ and ultimately improve systemic tissue perfusion.
4.3 Mechanical ventilation and respiratory measures
Systemic SaO2 saturation in TGA depends on the relative proportions and O2 saturation levels of the two sources of the systemic circulation, i.e. fully saturated pulmonary venous blood that is shunted from the pulmonary to the systemic circulation (‘effective’ systemic flow) and the systemic mixed venous blood that recirculates through the systemic vascular bed. The degree of intercirculatory mixing is dictated by the number, size and site of anatomical communications between the two circuits. The haemoglobin level is also important, and a level of around 15 g/dl is considered optimal. Systemic and pulmonary vascular resistances (PVR) add to the complex interplay of the preceding factors [Reference Mair and Ritter69, Reference Shaher70]. Preoperative manipulation of mixing and the other contributing factors should result in an O2 saturation level of 75–85% of the arterial blood gas. In preterm newborns, the lower end of the acceptable range can be as low as 70%. One important point is that the accuracy of pulse oximetry values <80% is limited in neonates [Reference Shiao and Ou71] and frequent monitoring of arterial blood gases may be warranted.
Neonates with profound hypoxaemia (partial pressure of arterial oxygen <25 mmHg and/or SaO2 <60%) require urgent attention [Reference Jouannic, Gavard, Fermont, Le Bidois, Parat and Vouhé35].
Conservative measures to increase systemic O2 saturation levels and adequate tissue oxygen delivery include (i) continuous PGE1 infusion to maintain ductal patency and emergent BAS to increase intercirculatory mixing; (ii) mild hyperventilation and increased fraction of inspired oxygen (FiO2) to lower PVR and increase pulmonary blood flow; (iii) transfusion to treat relative anaemia and increase O2-carrying capacity; (iv) sedation and paralysis to decrease O2 consumption; and (v) possibly inotropic support to increase cardiac output and O2 delivery [Reference Marino, Bird and Wernovsky72].
4.4 Nutrition
Infants with TGA and adequate intercirculatory mixing, without PGE1 infusion (e.g. after septostomy), should be fed enterally and encouraged to bottle-feed and breast-feed [Reference Howley, Kaufman, Wymore, Thureen, Magouirk and McNair73–Reference Sables-Baus, Kaufman, Cook and da Cruz75]. There is still great controversy surrounding the best approach to enteral nutrition for infants with cyanotic congenital heart defects, especially during the time when they are prostaglandin-dependent [Reference Howley, Kaufman, Wymore, Thureen, Magouirk and McNair73]. Based on limited evidence in favour or against the practice of feeding infants enterally while they are on PGE1 [Reference Willis, Thureen, Kaufman, Wymore, Skillman and da Cruz74, Reference Davis, Davis, Cotman, Worley, Londrico and Kenny76, Reference Natarajan, Reddy Anne and Aggarwal77], haemodynamically stable newborns with TGA should be fed enterally as soon as it is deemed feasible preoperatively, even while they are on PGE1. Breast milk and breast-feeding are preferred. Trophic enteral feeding may be considered in some patients in order to reduce the risk of translocation.
4.5 Major prematurity and very low birth weight
The incidence of low birth weight among newborns with TGA is reported to be 3.05% [Reference Soongswang, Adatia, Newman, Smallhorn, Williams and Freedom78], which compares favourably with the reported 15% overall incidence of prematurity or low birth weight in neonates with congenital heart disease [Reference Ades, Johnson and Berger79]. Although it is clear that low birth weight and prematurity are different factors, they often coexist. Low birth weight (≤2.5 kg), very low birth weight (≤1.5 kg) and, less so, prematurity [Reference Hickey, Nosikova, Zhang, Caldarone, Benson and Redington80] present technical and physiological challenges to complete repair in the neonate. Additional comorbidities from other organ systems (central nervous system, renal, gastrointestinal) increase the morbidity and mortality rates of these infants both short and long term [Reference Reddy81]. More specifically, in transposition, large, multi-institutional studies in Europe and North America have demonstrated increased mortality rates after an ASO in infants weighing <2.5 kg [Reference Curzon, Milford-Beland, Li, O’brien, Jacobs and Jacobs82, Reference Kansy, Tobota, Maruszewski and Maruszewski83]. However, it has been shown that delaying repair to allow for weight gain confers higher preoperative morbidity and early mortality without any associated benefit [Reference Reddy, McElhinney, Sagrado, Parry, Teitel and Hanley84, Reference Roussin, Belli, Bruniaux, Demontoux, Touchot and Planche85]. Furthermore, delaying intervention for TGA IVS results in deconditioning of the left ventricle, rendering the patient a potentially poor candidate for a primary ASO. Centres have reported early repair [Reference Azakie, Johnson, Anagnostopoulos, Egrie, Lavrsen and Sapru86], primary repair as late as age 3 months, late single-stage repair with postoperative mechanical circulatory support and two-stage repair (i.e. pulmonary artery banding with or without aortopulmonary shunt placement followed by an ASO in 7–14 days) [Reference Azakie, Johnson, Anagnostopoulos, Egrie, Lavrsen and Sapru86, Reference Rios, Dummer and Overman87] with acceptable results.

ASO: arterial switch operation; ECLS: extracorporeal life support; IV: intravenous; PGE1: prostaglandin E1; VAD: ventricular assist device.
a Class of recommendation.
b Level of evidence.
Recommendations for perinatal management in a neonatal intensive care unit
5. Surgery for Transposition of The Great Arteries With Intact Ventricular Septum
5.1 Timing for surgery of TGA IVS
The ASO for TGA IVS in newborns was introduced in the early 1980s. The assumption was that the neonatal left ventricle would be suited for systemic work after having withstood systemic pressure throughout foetal life [Reference Castaneda, Norwood, Jonas, Colon, Sanders and Lang88, Reference Quaegebeur, Rohmer, Ottenkamp, Buis, Kirklin and Blackstone89]. The neonatal ASO has since become the preferred approach for repair of TGA IVS and is currently achievable with an average surgical mortality rate of 2–5% [Reference Sarris, Chatzis, Giannopoulos, Kirvassilis, Berggren and Hazekamp90].
At birth, the left ventricular muscle mass is equivalent to that of the right ventricle. Subsequently, as a result of the rapid postnatal decrease in PVR, the left ventricle soon becomes ‘deconditioned’, losing muscle mass and the ability to function at systemic workloads [Reference Danford, Huhta and Gutgesell91, Reference Rudolph92]. Currently, the optimal timing for an ASO in babies with TGA IVS is established from the first few days to 3 weeks of life [Reference Duncan, Poirier, Mee, Drummond-Webb, Qureshi and Mesia93].
In Europe, 25–30% of patients with TGA IVS have undergone a routine ASO within the first week of life [Reference Sarris, Chatzis, Giannopoulos, Kirvassilis, Berggren and Hazekamp90]. It is worth noting that an ASO in the first few hours of life may obviate the need for BAS [Reference Chasovskyi, Fedevych, Vorobiova, Zhovnir, Maksimenko and Boychenko94, Reference Nevvazhay, Chernogrivov, Biryukov, Biktasheva, Karchevskaya and Sulejmanov95]. This very early approach, however, remains controversial.
Also, the upper age limit for a primary ASO in TGA IVS cannot be determined. Most surgeons undertake a primary ASO in babies up to 4 weeks of age, whereas the choice of a primary ASO beyond 1 month of age is controversial [Reference Sarris, Chatzis, Giannopoulos, Kirvassilis, Berggren and Hazekamp90]. In fact, several groups have electively adopted a primary ASO in late presenters (up to 8 weeks of age), planning postoperative mechanical support, if necessary [Reference Duncan, Poirier, Mee, Drummond-Webb, Qureshi and Mesia93, Reference Bisoi, Sharma, Chauhan, Reddy, Das and Saxena96–Reference Foran, Sullivan, Elliott and de Leval99], and accepting prolonged duration of postoperative ventilation and hospital stay [Reference Kang, de Leval, Elliott, Tsang, Kocyildirim and Sehic100]. A few outliers undergoing an ASO at up to 9 months of age have been reported [Reference Kang, de Leval, Elliott, Tsang, Kocyildirim and Sehic100, Reference D’Udekem, Cheung, Butt, Shann and Brizard101]. However, for infants older than 2 months, left ventricular mass and mass/end-diastolic volume ratio should, preferably, orient towards a rapid two-stage ASO.

ASO: arterial switch operation; ECLS: extracorporeal life support; IVS: intact ventricular septum; TGA: transposition of the great arteries.
a Class of recommendation.
b Level of evidence.
c References.
Recommendations for timing of the ASO
5.2 Adequacy of left ventricular myocardial mass
5.2.1 Left ventricular mass regression
Upon completion of a postnatal fall of pulmonary arteriolar resistance (around the fourth week of life), left ventricular mass in TGA IVS starts decaying [Reference Rudolph92, Reference Di Donato, Fujii, Jonas and Castaneda102], although with some degree of reversibility [Reference Bisoi, Malankar, Chauhan, Das, Ray and Das103, Reference Rudolph104].
In addition, isolated pulmonary outflow obstruction in TGA IVS, whether anatomical [Reference Bisoi, Sharma, Chauhan, Reddy, Das and Saxena96] or dynamic [Reference Lacour-Gayet, Piot, Zoghbi, Serraf, Gruber and Mace105, Reference Robinson, Wyse and Macartney106], may trigger left ventricular myocardial hypertrophy and potentially allow an ASO to be performed. Furthermore, a moderately restrictive ASD and a sizeable (≥5 mm) patent arterial duct may both preserve adequate left ventricular preload and pressure and partly explain the positive outcomes in some late presenters [Reference Kang, de Leval, Elliott, Tsang, Kocyildirim and Sehic100]. Finally, genetically predetermined factors might also account for the involution of PVR and left ventricular performance [Reference Bisoi, Sharma, Chauhan, Reddy, Das and Saxena96].
5.2.2 Assessment of left ventricular preparedness
Left ventricular ‘preparedness’ for an ASO is commonly judged on measurable parameters (e.g. left ventricular geometry, wall thickness and function on echocardiogram) and on additional evidence of pressure and volume loading related to the size of the duct and an ASD [Reference Danford, Huhta and Gutgesell91, Reference Kang, de Leval, Elliott, Tsang, Kocyildirim and Sehic100, Reference Lacour-Gayet, Piot, Zoghbi, Serraf, Gruber and Mace105, Reference Bano-Rodrigo, Quero-Jimenez, Moreno-Granado and Gamallo-Amat107–Reference Smith, Wilkinson, Arnold, Dickinson and Anderson110]. The ventricular septum is forged by unequal ventricular pressures and progressively shifts towards the pulmonary ventricle, assuming a banana-shaped appearance on an echocardiogram [Reference Iyer, Sharma, Kumar, Bhan, Kothari and Saxena111, Reference Sidi, Planche, Kachaner, Bruniaux, Villain and Le Bidois112]. The Marie Lannelongue group introduced echo-based markers to judge preparedness [Reference Lacour-Gayet, Piot, Zoghbi, Serraf, Gruber and Mace105]. On the contrary, the Great Ormond Street group found that, in the late ASO group, conventional measures of left ventricular pressure and function were not predictive of mortality or of the need for mechanical support [Reference Foran, Sullivan, Elliott and de Leval99]. Alternatively, left ventricular preparedness may be assessed by a ‘provocative’ pulmonary artery banding: If tolerated by the left ventricle for up to 15–30 min, a primary ASO is undertaken [Reference Dabritz, Engelhardt, von Bernuth and Messmer113].
5.3 Training of the left ventricle for a delayed arterial switch operation
5.3.1 Introduction and pathophysiological issues
In 1977, Yacoub et al. [Reference Yacoub, Radley-Smith and Maclaurin114] devised a two-stage approach for an ASO in older patients with TGA IVS that included a preparatory pulmonary artery banding together with a systemic-to-pulmonary shunt for left ventricular training, followed by an ASO several months later. However, this policy did not become widely adopted for frequent, intractable, postoperative, left ventricular dysfunction, probably because of the advanced age at pulmonary banding [Reference Borow, Arensman, Webb, Radley-Smith and Yacoub115, Reference Sievers, Lange, Onnasch, Radley-Smith, Yacoub and Heintzen116]. In 1989, Jonas et al. [Reference Jonas, Giglia, Sanders, Wernovsky, Nadal-Ginard and Mayer117] introduced the so-called rapid two-stage ASO, showing that left ventricular hypertrophy is elicited as early as 1–2 weeks after the imposition of a pressure load in younger patients beyond neonatal age.
An 85% increase in left ventricular mass within 5–7 days of applying a pulmonary artery band was demonstrated in infants with TGA IVS [Reference Jonas, Giglia, Sanders, Wernovsky, Nadal-Ginard and Mayer117, Reference Boutin, Jonas, Sanders, Wernovsky, Mone and Colan118]. Remarkably, both the capacity and the rapidity of left ventricular hypertrophy decrease with aging. The age limit at which the potential for myocyte hyperplasia in the human infant is lost is allegedly 3–6 months after birth [Reference Di Donato, Fujii, Jonas and Castaneda102].
5.3.2 Indications for a two-stage arterial switch operation
Categorical indications for left ventricular training include a combination of the following noninvasive criteria:
-
1. Indexed left ventricular mass <35 g/m2.
-
2. Age well above 3 weeks.
-
3. Ventricular septal profile, with a banana-like left ventricular shape on 2D echocardiograms.
-
4. Absence of a patent arterial duct or LVOT obstruction [Reference Lacour-Gayet, Piot, Zoghbi, Serraf, Gruber and Mace105].
Haemodynamic data may also be used, especially if BAS is achieved using heart catheterization rather than 2D echocardiographic guidance. Aortic and systemic venous oxygen saturations, right atrial pressure and the left/right ventricular pressure ratio may then be obtained [Reference Wernovsky, Giglia, Jonas, Mone, Colan and Wessel119]. In general, a left/right ventricular pressure ratio <0.6 is an indication for a staged ASO [Reference Dabritz, Engelhardt, von Bernuth and Messmer113, Reference Parker, Zuhdi, Kouatli and Baslaim120].
5.3.3 Types of left ventricular training and technical aspects
5.3.3.1 Hypoxic (pre-ASO) left ventricular training.
Hypoxic left ventricular training, often preceded by BAS, implies a two-stage ASO with preliminary imposition of either pressure or volume overload or, more commonly, combinations of both. Whichever of the three methods described below is used, a tolerable level of systemic O2 saturation and an adequate left ventricular preload must be sought.
-
1. Pulmonary artery banding combined with a systemic–pulmonary shunt (usually a modified Blalock–Taussig anastomosis) followed by an ASO after an interval that depends on the patient’s age [i.e. 1–2 weeks in young infants (rapid two-stage ASO) [Reference Lacour-Gayet, Piot, Zoghbi, Serraf, Gruber and Mace105, Reference Jonas, Giglia, Sanders, Wernovsky, Nadal-Ginard and Mayer117], or several months in older infants/children] [Reference Yacoub, Radley-Smith and Maclaurin114, Reference Parker, Zuhdi, Kouatli and Baslaim120–Reference Helvind, McCarthy, Imamura, Prieto, Sarris and Drummond-Webb122]. A moderate degree of both pressure and volume overload provides the most effective stimulus for ventricular hypertrophy, and a small-to-moderate ASD is advantageous to ensure the necessary volume preload for the left ventricle [Reference Ilbawi, Idriss, DeLeon, Muster, Gidding and Duffy123]. Through either a sternotomy or a thoracotomy, a systemic–pulmonary shunt is placed first, using a polytetrafluoroethylene (PTFE) vascular graft (size 3.5 mm). After opening the shunt, under an FiO2 of 30%, the pulmonary artery band is placed and, while directly monitoring the left ventricular pressure, sequentially tightened to obtain a left/right ventricular systolic pressure ratio of 0.7. Simultaneous 2D echocardiographic guidance provides information on the tightness of the banding: the occurrence of ventricular failure suggests that the banding is too tight. Postoperatively, patients are weaned from inotropes and mechanical ventilation, based upon echocardiographic documentation of sustained good left ventricular function [Reference Lacour-Gayet, Piot, Zoghbi, Serraf, Gruber and Mace105, Reference Helvind, McCarthy, Imamura, Prieto, Sarris and Drummond-Webb122]. In older patients, sequential tightening over several months may be necessary [Reference Bernhard, Yacoub, Regensburger, Sievers, Smith and Stephan124]. In the presence of a wide ASD for intercirculatory mixing, the single best predictor of SaO2 is the magnitude of pulmonary blood flow. Acute reduction of total pulmonary blood flow, as happens following pulmonary artery banding, drastically cuts both effective pulmonary and systemic flows [Reference Mair and Ritter69]. If no additional source of pulmonary blood flow is contemplated, the degree of banding must be mild enough to allow sufficient effective pulmonary blood flow. In these cases, a slower myocardial hypertrophic response should be expected.
-
2. Pulmonary artery banding combined with induced patency of the arterial duct, obtained either by prostaglandin infusion or by ductal stenting, depending on the anticipated duration of the interim period.
-
3. Induced patency of the arterial duct alone, obtained using either prostaglandin infusion or ductal stenting [Reference Sivakumar, Francis, Krishnan and Shahani125]. In this case it may be advisable NOT to pursue a wide ASD, to assure adequate preload of the left ventricle [Reference Harinck, Van Mill, Ross and Brom126]. Simple ductal stenting, or supposedly prolonged prostaglandin infusion, may also rapidly train the involuted left ventricle of late presenters within days to a few weeks [Reference Foran, Sullivan, Elliott and de Leval99, Reference Ilbawi, Idriss, DeLeon, Muster, Gidding and Duffy123, Reference Harinck, Van Mill, Ross and Brom126]. It can be a less morbid method of left ventricular training because it avoids haemodynamic stress, pulmonary artery distortion and neoaortic valve regurgitation. The use of moderate-sized (3.5 or 4 mm) coronary stents was suggested to avoid post-procedure heart failure [Reference Sivakumar, Francis, Krishnan and Shahani125].
5.3.3.2 Normoxic (post-ASO) left ventricular training.
Normoxic left ventricular training is adopted after an ASO presenting intraoperative left ventricular failure unrelated to a coronary problem. It may be achieved pharmacologically or by mechanical circulatory support (see section 6.5.1).
5.3.4 Results of left ventricular training for a delayed arterial switch operation
The reported risk of mortality after Stage I is very low and easily avoided by emergency takedown of the pulmonary artery banding [Reference Lacour-Gayet, Piot, Zoghbi, Serraf, Gruber and Mace105, Reference Iyer, Sharma, Kumar, Bhan, Kothari and Saxena111]. The initial postoperative course of these patients, however, is often characterized by significant morbidity associated with low-output syndrome of variable severity and significant metabolic acidosis [Reference Boutin, Wernovsky, Sanders, Jonas, Castaneda and Colan127–Reference Wernovsky, Wypij, Jonas, Mayer, Hanley and Hickey129]. A less tight pulmonary banding, with a left/right ventricular peak pressure ratio at 0.65, prevents left ventricular dysfunction while endorsing ventricular remodelling [Reference Lacour-Gayet, Piot, Zoghbi, Serraf, Gruber and Mace105, Reference Ilbawi, Idriss, DeLeon, Muster, Gidding and Duffy123].
The left/right ventricular pressure ratio increases from 0.5 before pulmonary banding to 1.0 before the ASO. Most of the increase in left ventricular mass (95%) occurs in the first week, with the most rapid rate of hypertrophy by Day 2 and an exponential fall in the growth rate thereafter. The left ventricular volume also progressively increases, but not as rapidly as the left ventricular mass, with a consequent gradual rise in left ventricular mass/volume ratio without acute dilation. The left ventricular ejection fraction is significantly reduced at 12 h after banding but returns to basal levels by 3.5 days after banding as compensatory hypertrophy takes place [Reference Iyer, Sharma, Kumar, Bhan, Kothari and Saxena111, Reference Boutin, Jonas, Sanders, Wernovsky, Mone and Colan118].
The early mortality rate after a rapid two-stage ASO is between 0 and 6%, and the postoperative course can be smoother than in a single-stage ASO due to the excellent left ventricular mass developed [Reference Lacour-Gayet, Piot, Zoghbi, Serraf, Gruber and Mace105, Reference Iyer, Sharma, Kumar, Bhan, Kothari and Saxena111, Reference Jonas, Giglia, Sanders, Wernovsky, Nadal-Ginard and Mayer117, Reference Ilbawi, Idriss, DeLeon, Muster, Gidding and Duffy123]. The late follow-up of the two-stage approach has revealed impaired left ventricular systolic performance [Reference Boutin, Wernovsky, Sanders, Jonas, Castaneda and Colan127], increased incidence of neoaortic regurgitation [Reference Colan, Boutin, Castaneda and Wernovsky128] and right ventricular outflow tract (RVOT) obstruction [Reference Wernovsky, Giglia, Jonas, Mone, Colan and Wessel119]. Nonetheless, most of these patients enjoy an excellent clinical condition and physical ability [Reference Lacour-Gayet, Piot, Zoghbi, Serraf, Gruber and Mace105].
5.3.5 Optimal time interval between stages
The key tool for surgical decision making after Stage I is 2D echocardiography [Reference Lacour-Gayet, Piot, Zoghbi, Serraf, Gruber and Mace105, Reference Iyer, Sharma, Kumar, Bhan, Kothari and Saxena111, Reference Jonas, Giglia, Sanders, Wernovsky, Nadal-Ginard and Mayer117]. Clinical and haemodynamic parameters are also important [Reference Wernovsky, Wypij, Jonas, Mayer, Hanley and Hickey129]. Proposed criteria for a safe second-stage ASO include left-to-right ventricular pressure ratio >0.85, left ventricular end-diastolic volume >90% of normal, left ventricular ejection fraction >0.5, posterior wall thickness >4 mm and a predictive wall stress <120×103 dynes/cm2 [Reference Nakazawa, Oyama, Imai, Nojima, Aotsuka and Satomi130]. At Marie Lannelongue, the ASO was performed when the left ventricular mass had reached 50 g/m2 [Reference Lacour-Gayet, Piot, Zoghbi, Serraf, Gruber and Mace105].
The median interval between Stages I and II is 10 days (range 5 days to 6 weeks) [Reference Lacour-Gayet, Piot, Zoghbi, Serraf, Gruber and Mace105, Reference Iyer, Sharma, Kumar, Bhan, Kothari and Saxena111, Reference Boutin, Jonas, Sanders, Wernovsky, Mone and Colan118]. Provided that adequate left ventricular mass and volume are rapidly achieved, the early Stage II ASO has the advantage of avoiding pericardial adhesions.

ASO: arterial switch operation; BAS: balloon atrial septostomy; IVS: intact ventricular septum; PTFE: polytetrafluoroethylene; TGA: transposition of the great arteries.
a Class of recommendation.
b Level of evidence.
c References.
Recommendations for left ventricular training
5.4 Surgical techniques and intraoperative surgical management
5.4.1 Intraoperative parameters and cardiopulmonary bypass
Cardiopulmonary bypass (CPB) policy regarding temperature, flow, the use of vasodilators and pH status management varies widely without a clear consensus among the members of the surgical community and with little evidence to justify endorsing one approach over another. Compared with low flow bypass, a deep hypothermic circulatory arrest (DHCA) strategy in infancy is associated with worse neuro-developmental outcomes. DHCA should, therefore, be avoided whenever possible, due to both early and late unfavourable impacts [Reference Bellinger, Wypij, duPlessis, Rappaport, Jonas and Wernovsky131, Reference Karl, Hall, Ford, Kelly, Brizard and Mee132].
Cannulation for CPB varies according to preference. The ascending aorta is typically cannulated just proximally to the innominate artery, and venous return occurs through a single atrial basket. Direct or indirect bicaval cannulation is a valid alternative, making intracardiac procedures more flexible without circulatory arrest. No evidence favours any of the methods. A properly placed systemic vent enhances visibility.
Myocardial protection strategies vary widely, from cold crystalloid to multidose cold blood cardioplegia, used antegradely through the aortic root and then directly through the coronary ostia. Recently, warm bypass strategies and warm blood cardioplegia were introduced with claimed advantages; however, none of the myocardial protection methods has yet been shown to be preferable.
Weaning off bypass should be straightforward unless there is ischaemia (related to any coronary transfer occurrence), left ventricular dysfunction due to ischaemia or ventricle detraining. Less commonly, problems with RVOT reconstruction or transient pulmonary hypertension may create weaning difficulties but are easily recognized.
Immediate postoperative targets are a stable ECG, with no electrical instability (suggesting ischaemia), left atrial pressure 5–15 mmHg, with evidence of good tissue perfusion and urine output >1 ml/kg/h. High lactate levels and ongoing acidosis are poor prognostic markers and require active corrective measures [Reference Charpie, Dekeon, Goldberg, Mosca, Bove and Kulik133, Reference Munoz, Laussen, Palacio, Zienko, Piercey and Wessel134].
There is no direct evidence to suggest that routine use of milrinone (a phosphodiesterase inhibitor) following an ASO improves outcome but it is recommended from extrapolation to its use for other infant cardiac procedures. It is recommended that milrinone be started for any patient with signs or symptoms of low cardiac output and with at least a left atrial pressure >15 mmHg. The recommended loading dose of milrinone is 50 µg/kg over 30–60 min, followed by an infusion of 0.375–0.750 µg/kg/min. If hypotension develops, blood pressure support with other inotropic/vasopressor agents (epinephrine or dopamine) may be necessary [Reference Hoffman, Wernovsky, Atz, Kulik, Nelson and Chang135].
Bleeding is a well-known problem after an ASO in neonates. Perfect suture lines are essential to prevent bleeding, but the use of antifibrinolytic drugs is common. Formerly, aprotinin in different doses and regimens proved to be effective and safe for kidney function [Reference Bojan, Vicca, Boulat, Gioanni and Pouard136]. Both epsilon-aminocaproic acid and tranexamic acid were introduced; tranexamic acid has the same level of safety and similar level of efficacy as epsilon-aminocaproic acid and is associated with improved outcomes. The use of prophylactic antifibrinolytic agents for ASOs should be considered [Reference Pasquali, Li, He, Jacobs, O’brien and Hall137]. The use of recombinant factor VII (rVII) was recently introduced and might be a valuable addition in cases with severe bleeding. However, scientific proof for the use of rVII is lacking.
Acute renal failure is prevalent after an ASO. However, prophylactic use of a peritoneal dialysis catheter is not recommended [Reference Bellinger, Wypij, duPlessis, Rappaport, Jonas and Wernovsky131].
Delayed sternal closure after an ASO has been a routine technique for many surgical groups over the years. Studies do not support the hypothesis that elective delayed sternal closure will reduce the morbidity after an ASO in neonates but they do confirm the safety and efficacy of the procedure.
5.4.2 Coronary transfer
All coronary artery patterns are theoretically transferable [Reference Quaegebeur, Rohmer, Ottenkamp, Buis, Kirklin and Blackstone89, Reference Kirklin, Blackstone, Tchervenkov and Castaneda138]. Coronary artery patterns have been identified as a risk factor for mortality in ASOs [Reference Sarris, Chatzis, Giannopoulos, Kirvassilis, Berggren and Hazekamp90, Reference Prêtre, Tamisier, Bonhoeffer, Mauriat, Pouard and Sidi139–Reference Wong, Finucane, Kerr, O’donnell, West and Gentles146]. Different techniques have been used for coronary transfer: the button technique in which coronary ostia are transferred to punch holes, and tissue is removed from the neoaortic root, to accommodate the ostia; the slit technique in which slit openings that may be linear or U-shaped are created in the neoaorta; and the trap-door technique in which L-shaped incisions are created, leaving a hinged flap, with an angle that seems to favour coronary transfer and improves landing. None of these techniques was demonstrated as being superior [Reference Bove147, Reference Villafane, Lantin-Hermoso, Bhatt, Tweddell, Geva and Nathan148]; however, the trap-door technique is recognized for reducing angulation for all cases, particularly for double-loop patterns.
In some specific cases, pericardium hoods allow for more flexibility and safer transfer [Reference Parry, Thurm and Hanley149, Reference Tireli, Korkut and Basaran150].
Large coronary buttons should be harvested, often requiring removal of most of the respective sinus of Valsalva. For eccentrically situated ostia, the nearest aortic valve commissure may need to be taken down and later resuspended in the neopulmonary root. The proximal segments of the coronary artery should be mobilized adequately to allow kink-free, tension-free and torsion-free translocation.
The coronary ostia are to be transferred to the respective facing sinuses, preferably laterally, and should not be compressed by the facing neopulmonary trunk, upon distension. The location on the sinuses is dictated by anatomical details, namely commissural placement. In most cases, surgeons prefer to place the left coronary button rather low, whereas the right coronary button needs to be placed higher. In some cases, namely posteriorly looping patterns, the right coronary artery button in pattern D is better implanted into a higher position, above the sinotubular junction and the main vessel suture line.
A single-ostium coronary pattern is a specific transfer challenge. It may or may not be associated with an intramural course of the proximal coronary artery, which may involve a commissural area. The two standardized techniques are the Yacoub technique [Reference Yacoub and Radley-Smith151] and the Imai technique [Reference Koshiyama, Nagashima, Matsumura, Hiramatsu, Nakanishi and Yamazaki152]. In the Yacoub technique, the single coronary ostium is harvested with a large aortic cuff, mobilizing the adjacent commissure if necessary. The ostium is then anastomosed to the posteriorly facing neoaorta, along its superior border, the pouch being completed by the distal end of the aorta, which is cut obliquely, leaving a long anterior lip. Alternatively, a pericardial patch can be used to augment the aortic suture line to accommodate the pouch. In this case, the ostium is not rotated more than 90°, allowing no torsion. This method of transfer is applied to a single-ostium Yacoub type B classification and also to a Yacoub type C in which the two coronary ostia are very close to each other and truly impossible to split apart. Alternatively, the Imai technique can be used: the ostium, or closely lying ostia, is left untouched without harvesting or without any rotation; the aortic wall above the ostium is excised to within 1–2 mm of the ostium; and a window opening is created to the adjacent neoaortic root. A small semilunar patch of pericardium or adjacent aortic tissue as a rotated flap of the non-coronary sinus is sutured to the inferior edges of the coronary button, creating a wide pouch. The reconstruction is completed by the ascending aortic suture line to the superior end of the pouch [Reference Koshiyama, Nagashima, Matsumura, Hiramatsu, Nakanishi and Yamazaki152]. None of these alternative methods was found to be superior; however, their use is recommended for single or ‘too-close’ ostia transfer [Reference Qamar, Goldberg, Devaney, Bove and Ohye153–Reference Mee159].
An intramural course often occurs in the presence of a single ostium but may occur with other complex patterns, also involving one or two main coronary arteries. Whenever it is detected, an intramural course should be addressed by unroofing, even when a commissure is involved. In this case the commissural area must be repaired. After unroofing, the coronary ostia are to be transferred normally using a trap-door technique [Reference Thrupp, Gentles, Kerr and Finucane144, Reference Asou, Karl, Pawade and Mee160, Reference Chen, Cui, Chen, Yang, Cui and Xia161].
Transferring coronary arteries in the presence of non-facing great arteries, i.e. in pure side-by-side vessel arrangements, is extremely challenging. Individualized techniques are recommended, i.e. using extensive proximal coronary artery mobilization, trap doors and even pericardium tube extensions, for anterior loops with distant lateralized vessels. The use of autologous pericardial hoods is sometimes useful to accommodate unexpected anatomical problems.
For experienced surgeons, all coronary artery patterns are said to be transferrable. However, there may be occasions in which the only safe option is to opt for the atrial switch. These cases are exceptional and are not recommended by any level of evidence or recommendation.
5.4.3 Right ventricular outflow tract reconstruction
The reconstruction of the neopulmonary trunk connecting the former aorta to the pulmonary bifurcation depends on the construction of a tension-free anastomosis, in order not to create any coronary compression and trying to minimize the risk of tension-induced late RVOT stenosis. This goal requires several manoeuvres: full mobilization of pulmonary arterial branches as far as the origin of the first pulmonary branching into the lung hilum; reconstruction of pulmonary tissue defects related to excision of the Valsalva sinuses area; and anterior or more lateral pulmonary bifurcation displacement, which is achieved mainly by the Lecompte manoeuvre.
The Lecompte manoeuvre [Reference Lecompte, Zannini, Hazan, Jarreau, Bex and Tu162], by which the pulmonary bifurcation is brought anterior to the aorta, should be performed routinely [Reference Delmo Walter, Miera, Nasseri, Huebler, Alexi-Meskishvili and Berger163–Reference Raju, Burkhart, Durham, Eidem, Phillips and Li169] whenever the aorta and pulmonary arterial trunks are orientated totally, or predominantly, anteriorly–posteriorly.
Whenever great vessels are located side by side, and depending on special orientations, the Lecompte manoeuvre may or may not be performed [Reference Chen, Cui, Chen, Yang, Cui and Xia161, Reference Hutter, Kreb, Mantel, Hitchcock, Meijboom and Bennink167, Reference Karl, Cochrane and Brizard170, Reference Lalezari, Bruggemans, Blom and Hazekamp171]. In cases where it is not performed, it is necessary to shift the pulmonary artery bifurcation, which is done by sliding the anastomosis to one of the pulmonary branches.
The pulmonary artery tissue defects related to coronary transfer must be filled in. Several patch materials and patching techniques may be used [Reference Delmo Walter, Miera, Nasseri, Huebler, Alexi-Meskishvili and Berger163, Reference Ullmann, Gorenflo, Bolenz, Sebening, Goetze and Arnold166, Reference Kawata, Kishimoto, Iwai, Ishimaru, Saito and Kayatani168, Reference Karl, Cochrane and Brizard170, Reference Imoto, Kado, Asou, Shiokawa, Miyake and Yasuda172–Reference Sakurai, Maeda, Miyahara, Nakayama, Murayama and Hasegawa174]; however, autologous pericardial patch material is used most widely [Reference Delmo Walter, Miera, Nasseri, Huebler, Alexi-Meskishvili and Berger163, Reference Ullmann, Gorenflo, Bolenz, Sebening, Goetze and Arnold166, Reference Sakurai, Maeda, Miyahara, Nakayama, Murayama and Hasegawa174]. Most surgeons use fresh pericardium [Reference Hutter, Kreb, Mantel, Hitchcock, Meijboom and Bennink167, Reference Rudra, Mavroudis, Backer, Kaushal, Russell and Stewart173], because the use of materials pretreated with glutaraldehyde, irrespective of its concentrations, is associated with the development of RVOT stenosis postoperatively [Reference Sakurai, Maeda, Miyahara, Nakayama, Murayama and Hasegawa174].
The so-called trousers patch reconstruction technique is generally used [Reference Delmo Walter, Miera, Nasseri, Huebler, Alexi-Meskishvili and Berger163, Reference Hutter, Kreb, Mantel, Hitchcock, Meijboom and Bennink167, Reference Rudra, Mavroudis, Backer, Kaushal, Russell and Stewart173], because it apparently produces less residual RVOT obstruction [Reference Delmo Walter, Miera, Nasseri, Huebler, Alexi-Meskishvili and Berger163].
In the so-called button hole technique for coronary harvest and transfer, the neopulmonary trunk may reconstructed using direct anastomosis [Reference Swartz, Sena, Atallah-Yunes, Meagher, Cholette and Gensini165, Reference Rudra, Mavroudis, Backer, Kaushal, Russell and Stewart173]; the punch holes should be filled in with patch material.
No evidence has been produced regarding the use of any particular suture material. Resection of a small segment of ascending aorta before aortic reanastomosis in order to allow a more tension-free pulmonary reconstruction has been proposed [Reference Raju, Burkhart, Durham, Eidem, Phillips and Li169].
No evidence regarding the use of glue, systematically or sporadically, to prevent bleeding, is available, although its use has become generalized.
5.4.4 Atrial septal defect in TGA IVS
Patients under consideration for an ASO always have an ASD: a secundum ASD, a patent foramen ovale or an ASD after septostomy. This defect is normally closed, either directly or by patch. As part of the procedure, a residual defect may be left intentionally, to make the postoperative course smoother, particularly in patients in whom PVR might fluctuate and prompt a haemodynamic crisis. However, there is insufficient evidence to make a recommendation in favour or against this practice.

ASO: arterial switch operation; DHCA: deep hypothermic circulatory arrest; ECG: electrocardiogram.
a Class of recommendation.
b Level of evidence.
c References.
Recommendations for management of cardiopulmonary bypass

ASO: arterial switch operation; RVOT: right ventricular outflow tract.
a Class of recommendation.
b Level of evidence.
c References.
Recommendations on surgical technique
6. Perioperative and postoperative management
6.1 Anaesthetic management
Pertinent preoperative and perioperative issues that require special attention include the following:
-
1. Cyanosis, which may delay induction of anaesthesia with inhaled anaesthetics: neonates with a high haematocrit and excessive viscosity may have impaired microvascular perfusion, outweighing the advantages of increased oxygen-carrying capacity. Reduction of red blood cell (RBC) volume is not recommended in this situation.
-
2. Elevated PVR: special attention must be paid to patients with high PVR who present as ‘poor mixers’ and require urgent surgery with a suboptimal acid–base balance.
-
3. Coexisting diseases: these could preclude the use of monitoring options [transoesophageal echocardiogram (TOE)].
-
4. Family rapport and parent informed consent: to help the family develop a sense of trust and a positive hospital experience, it is important to discuss with them line placement, prolonged ventilation and instrumentation issues.
6.1.1 Monitoring
Noninvasive monitoring for an ASO should include pulse oximetry (usually two sites, pre- and post-ductal, are used), five-lead ECG, end-tidal capnography, oxygen and anaesthetic gas analysis, automated blood-pressure-measurement cuff, multiple-site (rectal, tympanic or posterior pharyngeal) temperature measurement, volumetric urine collection and a precordial stethoscope during induction. In the presence of cyanosis, pulse oximetry overestimates SaO2 and is exacerbated with further decrease of partial pressure of arterial oxygen (PaO2) [Reference Fanconi176]. The partial pressure of end-tidal CO2 value is less reflective of partial pressure of arterial carbon dioxide (PaCO2) because of ventilation perfusion mismatching [Reference Lazzell and Burrows177]. Rectal and tympanic temperature readings overestimate brain temperature [Reference Stone, Young, Smith, Solomon, Wald and Ostapkovich178]. NIRS has been used routinely to evaluate both cerebral (forehead) [Reference Gottlieb, Fraser, Andropoulos and Diaz179] and tissue (renal) perfusion. Although more data in humans are needed, the use of noninvasive monitoring is likely to improve perioperative management in patients undergoing an ASO [Reference Tortoriello, Stayer, Mott, McKenzie, Fraser and Andropoulos180]. These technologies are useful indicators of trends in oxygenation [Reference Andropoulos, Stayer, Diaz and Ramamoorthy181].
Invasive monitoring includes placement of an arterial line and catheterization of a central vein for central venous pressure (CVP) measurement.
Transoesophageal echocardiogram is an invaluable perioperative tool for monitoring ventricular performance and evaluating surgical results. Attention should be paid to complications that could occur during the use of TOE, especially haemodynamic compromise from left atrial compression.
Blood-gas analysis, along with the ability to perform onsite thromboelastography and platelet count, is as important as haemodynamic monitoring for this procedure.
6.1.2 Induction and maintenance of anaesthesia
No studies support the use of any particular anaesthetic agents for induction and maintenance of anaesthesia. Patients usually present with established IV access. In such cases, an opioid-based anaesthetic is supported. All other IV induction agents could be used at judicious doses, once their primary effects on the myocardium and vascular system are carefully evaluated. In cases of the inhalation-induction technique, administration of sevoflurane, the preferred anaesthetic agent, should be done with care and not in ‘high’ inspired concentrations, which could lead to relative bradycardia and decreased systemic vascular resistance.
All inhaled anaesthetic agents are thought to offer a degree of ischaemic preconditioning not only to the myocardium but also to the brain [Reference Codaccioni, Velly, Moubarik, Bruder, Pisano and Guillet182] and kidney [Reference Lee, Ota-Setlik, Fu, Nasr and Emala183].
Administration of amnesia-inducing agents is frequently minimized, because the importance of perioperative recall in this age group is frequently underestimated. Benzodiazepines given intravenously or a volatile anaesthetic agent administered through a vaporizer on CPB should be considered.
6.2 Pre-cardiopulmonary bypass management
The duration of the pre-CPB period varies and requires the vigilance and intense involvement of the anaesthesiologist, particularly in periods when distractions are unavoidable (such as during line placement). It is important to realize that pre-CPB is the period when the parallel connection of the systemic and pulmonary circulations takes place, whereby adequate mixing is achieved by obtaining appropriate volume status. Systemic oxygenation is highly dependent on increased venous oxygen saturation (SvO2) and improves with volume administration. Some anaesthesiologists have a lower threshold for use of inotropic support during this period, because of its favourable potential impact on cardiac output and therefore oxygenation improvement. There are not enough data to support such a choice. The importance of optimizing PVR and ensuring adequate mixing should not be underestimated. In cases of late TGA corrections, decreased left ventricular performance is also important. Finally, during the pre-CPB period, attention should be paid to careful surgical manipulation of the aorta and vena cava because missteps at this point could precipitate arrhythmias, hypotension and blood loss, leading to further systemic desaturation.
6.3 Cardiopulmonary bypass and anaesthesia
The initiation of CPB introduces major changes in the pharmacokinetics and pharmacodynamics of administered agents. These changes, which are magnified by the volume of distribution changes in the neonate, require additional attention directed towards achieving adequate depth of anaesthesia [Reference Kussman, Zurakowski, Sullivan, McGowan, Davis and Laussen184] and enhancement of the effects of the administered vasoactive agents. Parameters of CPB management that require special anaesthesiological consideration include the following:
-
1. Haematocrit and blood product utilization on bypass. Use of blood products for an ASO is unavoidable and practice is institution specific. The majority of centres would add either whole blood or packed RBCs with fresh frozen plasma (FFP) to ensure a haematocrit ≥25%, especially during the cooling and rewarming phases when hypoxic and ischaemic brain injuries are most likely to occur [Reference Newburger, Jonas, Soul, Kussman, Bellinger and Laussen185]. The risk–benefit ratios favour the greater haematocrit approach up to 30%, which represents a shift from previous practice trends [Reference Wypij, Jonas, Bellinger, Del Nido, Mayer and Bacha186]. We propose the use of fresh blood up to 5 days old, because, compared with stored RBCs, fresh RBCs are more metabolically balanced, have a higher pH, contain less potassium and reduced concentrations of lactate [Reference Sumpelmann, Schurholz, Thorns and Hausdorfer187], lead to fewer pulmonary complications and renal dysfunction and have lower infection rates [Reference Mou, Giroir, Molitor-Kirsch, Leonard, Nikaidoh and Nizzi188].
-
2. Vascular tone. When an α-stat technique is used, catecholamine production and a relative alkaline environment lead to elevated systemic vascular resistance and not to homogeneous tissue and organ perfusion. The use of vasodilators, mainly α-receptor antagonists, is widely advocated. Phentolamine at 0.1–0.2 mg/kg, phenoxybenzamine, sodium nitroprusside and nitroglycerine offer vasodilating effects.
-
3. Systemic inflammatory response. Treatments intended to reduce the systemic inflammatory response to CPB and operative trauma in general include the use of steroids. The use of steroids has been questioned. Although many groups use steroids preoperatively in order to mitigate the inflammatory effects of CPB while providing myocardial protection [Reference Pasquali, Hall, Li, Peterson, Jaggers and Lodge189], various publications show that there are not enough evidence-based data to justify this practice [Reference Gessler, Hohl, Carrel, Pfenninger, Schmid and Baenziger190, Reference Graham, Atz, Butts, Baker, Zyblewski and Deardorff191]. A multicentre observational analysis performed in the USA and published in 2012 did not find any benefit associated with methylprednisolone in neonates undergoing heart surgery and suggested that increased infection occurred in certain subgroups [Reference Pasquali, Li, He, Jacobs, O’brien and Hall192]. Another recent systematic review of randomized controlled trials showed that, despite the demonstrated attenuation of CPB-induced inflammatory response following the administration of steroids and other potential clinical advantages (lower mortality rate and significant reduction of renal-function deterioration), a large prospective randomized study is still needed to verify clearly the effects of steroid prophylaxis in paediatric patients [Reference Scrascia, Rotunno, Guida, Amorese, Polieri and Codazzi193]. Studies of newer anti-inflammatory treatment modalities are under way, including the use of monoclonal antibodies for inflammatory products (i.e. complement, tumour necrosis factor and endotoxins), and these may offer advantages in the future. Currently, adequate heparinization, along with the use of coated circuits [Reference Miyaji, Hannan, Ojito, Jacobs, White and Burke194, Reference Boning, Scheewe, Ivers, Friedrich, Stieh and Freitag195], is recommended, because it offers evidence-based elimination of thrombin production, minimizing the degree of systemic inflammatory reaction [Reference Finley and Greenberg196]. Finally, it should be noted that the use of opioids (fentanyl at doses higher than 25 µg/kg) decreases stress response, modifies inflammation and stabilizes haemodynamics [Reference Hickey, Hansen, Wessel, Lang, Jonas and Elixson197]. The use of conventional and modified ultrafiltration techniques provides evidence of reduction of bypass-related perioperative morbidity. Both improve myocardial function, decrease tissue oedema and minimize ventilatory support times. Modified ultrafiltration does not necessitate the addition of crystalloids or colloids, as does conventional ultrafiltration, uses only the patient’s blood volume, but requires extra vigilance to avoid volume depletion and temperature alterations [Reference Liu, Ji, Long, Li and Feng198].
-
4. Glucose control. Although still controversial, glucose control should be kept in mind, to avoid extremes of blood-sugar levels. In the Boston Circulatory Arrest Study, intraoperative [Reference Wypij, Newburger, Rappaport, duPlessis, Jonas and Wernovsky199] and initial postoperative [Reference Ballweg, Wernovsky, Ittenbach, Bernbaum, Gerdes and Gallagher200] hyperglycaemia did not predict worse neuro-developmental outcomes.
6.4 Separation from cardiopulmonary bypass
Issues pertinent to ASOs that play important roles during separation from CPB are as follows:
-
1. Myocardial ischaemia resulting from either air emboli or stenotic coronary anastomoses. After the surgical coronary anastomotic result is evaluated, increasing the coronary perfusion pressure should resolve myocardial ischaemia. Elevated RV afterload after the Lecompte manoeuvre could be another cause of coronary ischaemia.
-
2. Labile pulmonary artery pressure and elevated PVR that require interventions.
-
3. Poor left ventricular performance, especially in late correction of TGA, requiring use of inotropic support (milrinone, epinephrine, dopamine or dobutamine) or mechanical circulatory support.
6.5 Transfer to the intensive care unit
Monitoring of O2 saturation; ECG; and end-tidal carbon dioxide (CO2), arterial, central venous and atrial pressures (if available), should be continuous when the patient is transferred from one area of the hospital to another.
Resuscitation drugs, airway equipment, blood products and fluids for intravascular volume replacement should also be available. Patients are transferred on high FiO2, with a manual resuscitator or preferably a Jackson-Rees circuit, which provides better clinical information for chest and lung compliance. For patients on NO, ensuring continuous administration of NO is required. Various battery charges should be checked as well as pacing availability. Once adequate depth of anaesthesia is assured, the endotracheal tube should be suctioned before arrival in the ICU.
Finally, but most importantly, communication and rapport with the ICU staff should be a top priority well before the patient is moved to the ICU. We recommend that ICU nursing staff be in the operating room before the patient is moved.

CPB: cardiopulmonary bypass; FFP: fresh frozen plasma; NIRS: near-infrared spectroscopy; RBCs: red blood cells.
a Class of recommendation.
b Level of evidence.
c References.
Recommendations for anaesthetic management
6.5.1 Postoperative management in the intensive care unit
Postoperative management of patients with TGA focuses on the optimization of cardiac output [avoidance and treatment of low cardiac output syndrome (LCOS)], tissue perfusion and respiratory status, and on mitigation of the stress response and inflammatory processes triggered by CPB. Early goals of management also include control of coagulopathy; management of vascular tone, anomalies and capillary leak; and prevention and management of total body volume overload.
6.5.1.1 Transition and handover from the operating room
Intensive-care management starts with an adequate, standardized and safe transfer from the operating room. Prior to transfer, a World Health Organization Surgical Safety Checklist should be applied [Reference Bergs, Hellings, Cleemput, Zurel, De Troyer and Van Hiel203]. The objectives are to have a face-to-face handover of information in a systematic, concise, cohesive and safe manner. The anaesthesiologist is responsible for the care of the patient until the report process is complete. The assessment of the patient by the cardiac ICU nurses and physicians must occur after the report process is complete [Reference Boat and Spaeth204–Reference Vergales, Addison, Vendittelli, Nicholson, Carver and Stemland209].
6.5.1.2 Haemodynamic and tissue perfusion monitoring
After the ASO, patients require noninvasive and invasive monitoring. Basic monitoring strategies include continuous ECG; respiratory rate; noninvasive blood pressure cuff; core and peripheral temperatures; systemic oximetry and invasive arterial blood pressure; CVP; inspired end-tidal capnography; and, infrequently, continuous left atrial pressure. The acid–base status, mixed venous O2 saturations and serum lactate levels are excellent markers of O2 delivery [Reference Seear, Scarfe and LeBlanc210]. Patients may also be monitored with NIRS, which is a good surrogate marker of mixed venous oxygen saturation and hence a good continuous monitor of cardiac output and O2 delivery [Reference Bhutta, Ford, Parker, Prodhan, Fontenot and Seib211, Reference Mittnacht212]. Alternative technologies include invasive continuous mixed venous saturation monitoring and devices based on arterial wave contour analysis. Urine output must be carefully monitored although it is a poor early indicator of low cardiac output and deficient oxygen delivery.
6.5.1.3 Ancillary evaluation
Baseline evaluation includes a chest radiograph, arterial blood gas with a lactate level, central mixed venous saturation if required (in unstable patients), basic electrolyte and renal function panels and coagulation screening. Patients with bleeding may need more frequent evaluation of coagulation and possibly thromboelastograms, which would direct a goal-orientated therapy. Echocardiography is indicated for all unstable patients. Awareness of signs of coronary insufficiency is of utmost importance. An immediate postoperative ECG is routinely obtained and compared with preoperative evaluations. In the event of electrocardiographic or clinical evidence of ischaemia, nitroglycerine may be started; an echocardiograph, emergency catheterization and immediate surgical revision should be considered.
6.5.1.4 Haemodynamic management
Proper haemodynamic support must be based on comprehensive haemodynamic and pathophysiological appraisals [Reference Holmes213–Reference Zaritsky and Chernow215].
The most important take-home message is that the ultimate objective is to provide adequate tissue perfusion. Mean arterial pressures of 35–45 mmHg are acceptable for newborns following an ASO. Left atrial pressures, when monitored, should be maintained in the 8–10 mmHg range because volume boluses to increase this pressure are not well tolerated by the non-compliant left ventricle. The presence of right ventricular hypertension is unusual, and its presence should be promptly investigated using echocardiography. Residual intracardiac shunts or branch pulmonary artery stenosis following the Lecompte manoeuvre have both been implicated in elevated right ventricular pressures [Reference Stoica, Carpenter, Campbell, Mitchell, da Cruz and Ivy7, Reference Deshpande, Wolf, Kim and Kirshbom216]. Neonates undergoing an ASO receive milrinone (0.5–1.0 µg/kg/min) and low-dose dopamine (3–5 µg/kg/min) or low-dose adrenaline (0.01–0.05 µg/kg/min) to augment ventricular function if needed. Calcium chloride (10%) infusions at 5–15 mg/kg/h may also be helpful. Systemic afterload reduction can also be achieved with numerous drugs that add to the vasodilatory effect of milrinone; these may be alpha-blockers (phentolamine, phenoxybenzamine) or sodium nitroprusside. Vascular tone may be an issue in patients who present with significant inflammatory reactions. Vasopressin, low-dose norepinephrine and phenylephrine at low doses are useful to antagonize such vasodilation, optimize target organ perfusion and eventually optimize urine output, while reducing fluid overload [Reference Burton, Kaufman, Goot and da Cruz217].
6.5.1.5 Management of rhythm and conduction disorders
Following an ASO, neonates may be vulnerable to arrhythmias, which may relate to various factors [Reference Decker, McCormack and Cohen218]. Arrhythmias are associated with increased mortality rates; therefore, anticipating risk factors and preventing arrhythmias remain important objectives during the postoperative course [Reference Decker, McCormack and Cohen218, Reference Shamszad, Moore, Ghanayem and Cooper219]. Many arrhythmias are easily solved. In patients undergoing an ASO, indwelling lines may cause arrhythmias triggered by irritation of the myocardium when their tip is in an intracardiac position, and in this case, they require removal [Reference Decker, McCormack and Cohen218, Reference Texter, Kertesz, Friedman and Fenrich220]. Usually automatic tachycardia and re-entry tachycardia may not be well tolerated in the early postoperative phase, particularly in patients with marginal haemodynamics. For patients with arrhythmias, priority should be given to immediate resuscitation efforts if they are haemodynamically unstable; parallel steps focus on the correction of electrolyte and acid–base disturbances, optimization of ventilation, repositioning of intrathoracic lines—general, simple measures that may resolve the arrhythmias. If these measures fail, prompt antiarrhythmic therapy should be initiated. One of the most common arrhythmias after an ASO is junctional ectopic tachycardia (JET). In the management of JET, the following measures are recommended: controlled hypothermia, decrease of vasoactive drugs, optimization of electrolytes, use of dexmedetomidine or amiodarone and pacemaker strategies. Ideally, for safety reasons, antiarrhythmic drugs should be titrated as continuous infusions. Esmolol [221, Reference Trippel, Wiest and Gillette222], procainamide [Reference Benson, Dunnigan, Green, Benditt and Schneider223–Reference Mandapati, Byrum, Kavey, Smith, Kveselis and Hannan225] and amiodarone [Reference Dubin224, Reference Coumel and Fidelle226–Reference Perry, Knilans, Marlow, Denfield, Fenrich and Friedman229] are the most commonly used antiarrhythmic drugs in the neonatal period [Reference Dubin224]. Other commonly used antiarrhythmic drugs used in the neonate after an ASO are sotalol [Reference Dubin224, Reference Maragnes, Tipple and Fournier230], propranolol [Reference Dubin224], digoxin [Reference Brugada, Blom, Sarquella-Brugada, Blomstrom-Lundqvist, Deanfield and Janousek231], adenosine and magnesium sulphate [Reference Decker, McCormack and Cohen218].
6.5.1.6 Low cardiac output syndrome
Low cardiac output syndrome is multifactorial, related to CPB and circulatory arrest or to myocardial preservation, mechanical disruption of the myocardium, postinflammatory effects of bypass, and coronary manipulation, among others [Reference Wernovsky, Wypij, Jonas, Mayer, Hanley and Hickey129, Reference Vernon and Witte232]. LCOS may be more likely to happen when surgical intervention is delayed. It is critical to anticipate subtle changes that may indicate the inception of LCOS, including urine output, clinical perception of peripheral perfusion, and trends in heart rate and pressures, but this awareness may be related to the caregivers’ experience [Reference Tibby, Hatherill, Marsh and Murdoch233]. Persistent systemic hypotension in the setting of poor peripheral perfusion, elevated left atrial pressure, elevated serum lactate or decreased cerebral NIRS and other signs of left ventricular dysfunction should be promptly investigated. Any suspicion of coronary insufficiency should be addressed immediately, with possible re-exploration and revision. In the absence of coronary insufficiency, low cardiac output can be caused by primary myocardial dysfunction. Pharmacological support is therefore provided with vasoactive, inotropic and lusitropic drugs titrated to improve cardiac output with systemic afterload reduction and to enhance tissue perfusion markers.
6.5.1.7 Extracorporeal life support
In certain cases, mechanical circulatory support with ECLS or a ventricular assist device (VAD) may be indicated. In situations where adequate haemodynamics cannot be achieved or can be achieved only with high inotropic support (exposing the patient to significant myocardial oxygen consumption and progressive lactic acidosis and progressive alteration of tissue perfusion markers), a brief period of ECLS may help to bridge the patients to recovery. In such scenarios, it is vital to rule out residual defects that may need surgical revision prior to weaning the patient from the ECLS. The need for ECLS has been reported in around 20% of infants undergoing an ASO beyond the sixth week of life [Reference Bisoi, Ahmed, Malankar, Chauhan, Das and Sharma234].
6.5.1.8 Sedation and analgesia.
All medications should be given following the principle of minimal effective dosing. Analgesic and sedative strategies should encompass the provision of baseline comfort and the limitations of haemodynamic side effects. No combinations have proven superior, but it is important to remain consistent. Universal pain and sedation scores (i.e. the COMFORT scale) are required and will be used to evaluate needs and drug titration [Reference Ambuel, Hamlett, Marx and Blumer235]. Avoidance of prolonged use may help prevent subsequent withdrawal symptoms. Strategies to try to avoid withdrawal may include drug rotation, daily interruption of infusions and the use of long-acting agents; nonetheless, the most effective method is to avoid using opioids and benzodiazepines for more than 5 days [Reference Rawlinson and Howard236].
The most commonly prescribed combinations include opioids and benzodiazepines. Morphine and fentanyl are the most frequently used opioids for maintenance and breakthrough analgesia. Continuous infusions may be better tolerated and allow easier titration to minimal effective doses. It is important to keep in mind that the combination with nonopioid analgesia (i.e. paracetamol) reduces the requirements for opioids. Midazolam, the most commonly used agent for sedation, provides effective sedation and amnesia. Dexmedetomidine is a newer agent that has been approved for use in the European Union since 2011; it produces stable sedation without respiratory depression and decreases the need for other sedatives or analgesics [Reference Chrysostomou, Morell, Wearden, Sanchez-de-Toledo, Jooste and Beerman237–Reference Tobias and Chrysostomou240]. The use of muscular relaxants should be avoided in the postoperative course of an ASO. It may occasionally be needed to promote ventilator synchrony with specific modalities. It may also be useful to induce hypothermia as part of the management of LCOS or after cardiac arrest, or in the event of pulmonary hypertension crisis or JET. Ventilated neonates on muscle relaxants have a higher than average overall mortality rate compared with neonates managed without muscle relaxants [Reference Martin, Bratton, Quint and Mayock241]. Neuromuscular blockade should be administered only in patients who are deeply sedated with appropriate monitoring [Reference Rawlinson and Howard236]. If necessary, its administration should be short-lived because it carries numerous inconveniences [Reference Tabarki, Coffinieres, Van Den Bergh, Huault, Landrieu and Sebire242]. Vecuronium is used most commonly; rocuronium and cisatracurium are also used frequently.
6.5.1.9 Ventilation and airway management.
Mechanical ventilation targets the maintenance of homeostasis (pH 7.35–7.45, PaCO2 35–45 mmHg, no hypoxaemia) while avoiding barotrauma. Ventilatory modalities and parameters need to be adapted to specific conditions such as pulmonary hypertension. Neonates with ongoing bleeding, unstable haemodynamics or rhythm abnormalities and those with delayed closure of the sternum should remain mechanically ventilated. All other patients should be weaned from mechanical ventilation over 12–24 h at the latest. Prevention of ventilator-associated pneumonia is essential in neonates who remain ventilated after the ASO. The main clinical bundles in the prevention recommendations are head-of-bed positioning, closed suction, oral care and patient repositioning [Reference Foglia, Meier and Elward243–Reference Elward, Warren and Fraser246].
6.5.1.10 Fluid and electrolyte management.
Fluid overload can have a profound influence on the initial postoperative course; therefore a methodical follow-up of fluid intake and output is essential. IV fluids with 10% dextrose are given at 50% maintenance (2 ml/kg/day) for the first 24 h and then gradually used more liberally after that time. Hypotension due to hypovolaemia is treated with 5–10 ml/kg bolus infusions of 5% albumin, normal saline or FFP. Patients who are tenuous and anaemic ought to receive concentrated RBCs, and patients with persistent bleeding may require FFP, platelets and packed RBCs. On Day 2 postoperatively, fluids may be increased to 75% of requirements and then to 100% from Day 3 onwards.
6.5.1.11 Renal management.
Transient renal insufficiency and acute kidney injury are fairly common and associated with increased morbidity and mortality [Reference Gil-Ruiz Gil-Esparza, Alcaraz Romero, Romero Otero, Gil Villanueva, Sanavia Moran and Rodriguez Sanchez de la Blanca247–Reference Watkins, Williamson, Davidson and Donahue251]. This disturbance is not exclusive to unstable patients and is multifactorial. A methodical evaluation of patients with suboptimal urine output is necessary and may require the estimation of a Paediatric Risk, Injury, Failure, Loss, End-Stage Renal Disease (pRIFLE) score. Furosemide is started 8–12 h after surgery (0.5–1 mg/kg boluses every 6–24 h or continuous infusion at 0.05–0.3 mg/kg/h).
6.5.1.12 Feeding and nutrition.
Enteral feeding is started when haemodynamics are stable. If enteral feeding cannot be started by Day 1 postoperatively, then total parenteral nutrition should be initiated. Mechanically ventilated patients can be fed via a nasogastric tube or a post-pyloric feeding tube if there is concern about gastro-oesophageal reflux. If the patient is breathing spontaneously and has stable respiratory mechanics, one can try oral feedings. In addition, ranitidine should be used prophylactically in the postoperative period.

ECG: electrocardiogram; ECLS: extracorporeal life support.
a Class of recommendation.
b Level of evidence.
c References.
Recommendations for intensive care postoperative management
7. Special topics
7.1 Atrial switch—is there a role for it?
7.1.1 Introduction
Over the years, atrial switch procedures have almost entirely yielded to the ASO in TGA IVS and are currently limited to rare indications. The long-term outcome following atrial switch procedures is reported to be 80% survival at 25–30 years [Reference Moons, Gewillig, Sluysmans, Verhaaren, Viart and Massin254–Reference Wilson, Clarkson, Barratt-Boyes, Calder, Whitlock and Easthope258]. There is a significant late hazard function affecting this population, and sudden death occurs in 6–17% of patients [Reference Wilson, Clarkson, Barratt-Boyes, Calder, Whitlock and Easthope258]. Sudden death may relate to the occurrence of atrial arrhythmias [Reference Birnie, Tometzki, Curzio, Houston, Hood and Swan259] and loss of sinus rhythm [Reference Sun, Happonen, Bennhagen, Sairanen, Pesonen and Toivonen260]. Furthermore, the long-term outcome of these patients may entail a number of reoperations or interventional procedures in 10–20% of survivors, most commonly for baffle leak or obstruction [Reference Gewillig, Cullen, Mertens, Lesaffre and Deanfield261] or tricuspid regurgitation [Reference Duncan and Mee262].
7.1.2 Current role of atrial switches
Possible indications for atrial switch procedures in patients with TGA IVS are given in the ‘Recommendations for atrial switch’ (see section 7.1.4 ‘Treatment for late failure of the systemic right ventricle’ section).
7.1.3 Who still knows how—and is able—to perform this operation?
The currently limited application of the Senning and Mustard operations has deprived the younger generations of congenital heart surgeons of sufficient exposure to the many tips and pitfalls of the atrial switch procedures. Yet, mastering these techniques can be crucial for special circumstances. Exhaustive references dealing with the technical aspects of these procedures are available for both the Senning [Reference Barron, Jones and Brawn263–Reference Senning265] and the Mustard [Reference Mustard266] operations, which are still indicated in conditions outside the scope of these guidelines.
7.1.4 Treatment for late failure of the systemic right ventricle
The treatment for late failure of the systemic right ventricle after the Senning or Mustard operation is controversial. The following two options are available:
-
1. Medical management and/or cardiac devices eventually followed by heart transplant, or
-
2. Conversion (staged) to anatomical repair.
7.1.4.1 Medical therapy for TGA patients with systemic right ventricular failure
Conservative management should include meticulous echocardiographic follow-up, with exercise testing when appropriate, to detect early changes in ventricular/valvular function and decrements in the patient’s functional status [Reference Duncan and Mee262]. Mild systemic right ventricular dysfunction with mild-to-moderate tricuspid regurgitation can be treated conservatively with afterload reduction and control of arrhythmias [Reference Duncan and Mee262, Reference Winter, Bouma, Groenink, Konings, Tijssen and van Veldhuisen267]. Afterload reduction, using angiotensin-converting enzyme inhibitors, β-blockers and diuretics, has proved useful in this setting [Reference Winter, Bouma, Groenink, Konings, Tijssen and van Veldhuisen267, Reference Hechter, Fredriksen, Liu, Veldtman, Merchant and Freeman268].
7.1.4.2 Cardiac devices for TGA patients with systemic right ventricular failure
Pacemaker implantation can restore physiological cardiac rhythm, with 11% of pacemaker dependency reported in the adult population of TGA patients [Reference Gelatt, Hamilton, McCrindle, Connelly, Davis and Harris269]. Electromechanical dyssynchrony, secondary to right bundle branch block or left ventricular pacing, may respond to cardiac resynchronization therapy [Reference Diller, Okonko, Uebing, Ho and Gatzoulis270, Reference Janousek, Tomek, Chaloupecky, Reich, Gebauer and Kautzner271]. Implantable cardioverter defibrillators may help prevent sudden death [Reference Triedman272]. The use of left VAD for RV failure following the Mustard operation has been described [Reference Kenleigh, Edens, Bates and Turek273].
7.1.4.3 Surgery for patients with TGA with right ventricular/tricuspid valve dysfunction
For cases with progressive right ventricular and tricuspid valve dysfunction, surgical therapy is indicated.
-
1. Tricuspid valve surgery: tricuspid valve repair or replacement may improve right ventricular function, but only if surgery is performed before significant right ventricular dysfunction ensues [Reference Warnes274]. It is unclear whether tricuspid valve replacement should be preferred to tricuspid valvuloplasty; however, surgical intervention is associated with a risk of early mortality (up to 10%) and the need for reoperation (in another 25%) [Reference van Son, Danielson, Huhta, Warnes, Edwards and Schaff275]. In general, tricuspid valve function and functional class improve significantly after surgery, and systemic right ventricular function is preserved [Reference Scherptong, Vliegen, Winter, Holman, Mulder and van der Wall276].
-
2. Pulmonary artery banding and conversion to anatomical repair: in the presence of reversible right ventricular dysfunction associated with well-maintained left ventricular and mitral valve function, conversion to anatomic correction may be considered.
However, with the exception of cases with long-standing pulmonary hypertension (e.g. for residual pulmonary venous obstruction) or ventricular outflow tract obstruction, the left ventricle must be retrained by incremental pulmonary artery banding [Reference Mavroudis and Backer277]. Gradual left ventricular training is vital because overzealous banding can induce left ventricular failure [Reference Poirier, Yu, Brizard and Mee278]. Interestingly, the increase in the left ventricular pressure may induce a rightward septal shift, with improved coaptation of the leaflets and consequent reduced regurgitation of the systemic atrioventricular valve. The result is reduced right ventricular volume load and improved function. Whether this approach can be considered a definitive palliation is not yet clear [Reference Duncan and Mee262, Reference Warnes274, Reference van Son, Danielson, Huhta, Warnes, Edwards and Schaff275, Reference Mavroudis and Backer277]. Nonetheless, it can act as a bridge to transplant [Reference Poirier, Yu, Brizard and Mee278].
7.1.4.4 Methods for conversion of atrial switch to an ASO
Left ventricular training in children or older patients is a much slower process than it is in infants and is achievable by different strategies of incremental pulmonary artery banding:
-
1. Single, long-standing (≥1 year) pulmonary artery banding, in which the patient gradually outgrows an initially loose band [Reference Poirier, Yu, Brizard and Mee278].
-
2. Multiple surgical procedures (2–3 stages) with progressive tightening of the band [Reference Poirier, Yu, Brizard and Mee278].
-
3. Application of a telemetric adjustable pulmonary artery band, undertaking a variety of cardiac fitness protocols [Reference Corno279, Reference Dibardino, Kleeman and Bove280].
7.1.4.5 Indications and timing for conversion of atrial switch to an ASO
There is an age-dependent time limit for preparatory pulmonary artery banding to recondition the left ventricle [Reference Di Donato, Fujii, Jonas and Castaneda102, Reference Helvind, McCarthy, Imamura, Prieto, Sarris and Drummond-Webb122, Reference Warnes274]. Clinically, the response to left ventricular training appears inconsistent among adolescents and adults [Reference Mavroudis and Backer277, Reference Poirier, Yu, Brizard and Mee278, Reference Padalino, Stellin, Brawn, Fasoli, Daliento and Milanesi281]. This response is a possible consequence of inadequate myocardial perfusion in the presence of suddenly increased cardiac work during pulmonary banding and induced myocardial hypertrophy [Reference Di Donato, Fujii, Jonas and Castaneda102, Reference Poirier, Yu, Brizard and Mee278]. In older patients, failure to achieve adequate reconditioning of the left ventricle increases the risk of death and calls for timely transplant [Reference Helvind, McCarthy, Imamura, Prieto, Sarris and Drummond-Webb122]. In general, however, strict contraindications for left ventricular reconditioning and/or ultimate anatomical correction do not exist [Reference Padalino, Stellin, Brawn, Fasoli, Daliento and Milanesi281].
7.1.4.6 Pulmonary artery banding—operative and postoperative management
The band is gradually tightened until a drop in the systemic blood pressure and a rise in the CVP are observed, together with a decrease in oxygen saturation or the development of rhythm disturbances; the band is then loosened slightly [Reference Duncan and Mee262, Reference Daebritz, Tiete, Sachweh, Engelhardt, von Bernuth and Messmer282]. This step usually corresponds to an increment in left ventricular pressure of 20–50 mmHg. TOE can identify any shift of the ventricular septum and reduction of tricuspid regurgitation and right ventricular dysfunction. Conversely, if TOE demonstrates any deterioration in left ventricular function or the manifestation of mitral regurgitation, the band should be loosened immediately [Reference Duncan and Mee262].
7.1.4.7 Interim follow-up during left ventricular training
Left ventricular function and pressures are periodically reassessed (e.g. every 3 months) using echocardiography, magnetic resonance imaging (MRI) and cardiac catheterization. Criteria for left ventricular preparedness include ventricular ejection fraction of ≥55%, less-than-mild mitral regurgitation, ventricular systolic pressure ≥80% of systemic blood pressure, ventricular end-diastolic pressure ≤10 mmHg, normal indexed ventricular mass (≥60 g/m2) and normal wall thickness [Reference Duncan and Mee262, Reference Poirier, Yu, Brizard and Mee278]. If these criteria are not met, pulmonary artery banding is repeated. Roughly one to two band-tightening procedures over a 1-year period may be needed [Reference Duncan and Mee262, Reference Poirier, Yu, Brizard and Mee278, Reference Benzaquen, Webb, Colman and Therrien283]. If left ventricular dysfunction is observed or if atrial arrhythmias progress, the banding is taken down.
Patients are listed for transplant when their condition deteriorates to end-stage heart failure [Reference Poirier, Yu, Brizard and Mee278].
7.1.4.8 Anatomical correction—operative and postoperative management
Along with take-down of atrial repair and conversion to anatomical correction [Reference Mee284], additional procedures may be needed to manage neoaortic insufficiency or atrial arrhythmias [Reference Mavroudis and Backer277]. The immediate postoperative therapy must comprise aggressive reduction of both pre- and afterload (using phenoxybenzamine and nitroprusside) and adequate inotropic support (using dopamine, dobutamine and milrinone). Nitroglycerine therapy may help to prevent coronary vasospasm and to control pulmonary artery vasoreactivity. Fluid challenges are contraindicated. Patients stay paralyzed and ventilated for at least 24 h, and chronic afterload reduction medication is started orally once the patient is extubated [Reference Mavroudis and Backer277].
7.1.4.9 Results for anatomic conversion of TGA after the mustard or senning operation.
The median duration of pulmonary artery banding for left ventricular training is 13 months (maximum duration up to 5 years). Approximately one-half of these patients survive after an ASO in New York Heart Association functional class I or II, whereas another one-quarter need a heart transplant [Reference Duncan and Mee262, Reference Mavroudis and Backer277, Reference Daebritz, Tiete, Sachweh, Engelhardt, von Bernuth and Messmer282, Reference Chang, Wernovsky, Wessel, Freed, Parness and Perry285, Reference van Son, Reddy, Silverman and Hanley286]. Additional morbidity occurs in these patients due to progressive aortic insufficiency and arrhythmias [Reference Mavroudis and Backer277, Reference Poirier, Yu, Brizard and Mee278].
7.1.4.10 Heart transplant.
Patients presenting with advanced right ventricular or biventricular failure, severe tricuspid or mitral dysfunction, pulmonary valve abnormalities precluding its use as a neoaortic valve, resilient arrhythmias or heart block, especially beyond adolescence, should not be offered left ventricle training measures with the ultimate intention of anatomical correction and should be enrolled in the heart transplant pathway [Reference Duncan and Mee262]. Nowadays, heart transplant is a well-established treatment strategy [Reference Hetzer, Weng and Delmo Walter287] and is likely to be a superior option to anatomical conversion from a functional aspect. However, it is challenging for both patient and clinician and, even more importantly, has limited application due to the shortage of donor organs [Reference Scherptong, Vliegen, Winter, Holman, Mulder and van der Wall276, Reference Canter, Shaddy, Bernstein, Hsu, Chrisant and Kirklin288]. Therefore, currently, the most reasonable approach for patients with late right ventricular failure after an atrial switch repair is probably to integrate left ventricular reconditioning and an anatomical correction protocol into a cardiac transplant programme that can serve as a bailout solution [Reference Poirier, Yu, Brizard and Mee278].

ASO: arterial switch operation; IVS: intact ventricular septum; TGA: transposition of the great arteries.
a Class of recommendation.
b Level of evidence.
c References.
Recommendations for atrial switch

a Class of recommendation.
b Level of evidence.
c References.
Recommendations for late left ventricular training
7.2 Cardiology follow-up protocols
Recommendations for follow-up of ASO patients are challenged by several factors: (i) current consensus on appropriate time interval and modality of surveillance imaging is still lacking; (ii) symptoms are rare and can be atypical, particularly in cases of coronary obstruction; (iii) a clear treatment strategy for sub-clinical anatomical or functional findings is not available; and (iv) during long-term follow-up, the effects of superimposed coronary artery disease on the operated coronary arteries are still unknown [Reference Villafane, Lantin-Hermoso, Bhatt, Tweddell, Geva and Nathan148].
Nevertheless, according to the common standard of care, short-term follow-up during the first year after an ASO should include clinical visits at 1, 3, 6 and12 months postoperatively. During mid- and long-term follow-up, children should be examined yearly and adults, every second year [Reference Wernovsky, Rome, Tabbutt, Rychik, Cohen and Paridon292].
Each visit should include history taking, clinical examination, 12-lead ECG and transthoracic echocardiography. A 24-h ECG should be performed annually in small children, because it may indirectly detect myocardial ischaemia. Regular exercise testing should be used to screen for myocardial ischaemia as soon as the patient is old enough to cooperate.
Well-known sequelae after an ASO include obstruction of the coronary arteries with related myocardial ischaemia, left ventricular dysfunction, stenoses of the pulmonary artery side branches, RVOT obstruction, neoaortic valve insufficiency and dilatation of the aortic root [Reference Tobler, Williams, Jegatheeswaran, Van Arsdell, McCrindle and Greutmann145, Reference Angeli, Raisky, Bonnet, Sidi and Vouhé293]. Therefore, follow-up should be targeted to detect such lesions.
Supravalvular pulmonary stenosis, particularly in the side branches of the pulmonary arteries, occurs mainly early after an ASO. Transthoracic echocardiography is the modality of choice for detecting RVOT stenosis. Additional imaging is frequently required, typically cardiac MRI, which has the advantages of providing clear anatomical delineation of the pulmonary arteries and additional functional information; therefore, cardiac MRI is considered the modality of choice for determining the need for a catheter-guided intervention involving the pulmonary arteries [Reference Valsangiacomo Buechel, Grosse-Wortmann, Fratz, Eichhorn, Sarikouch and Greil294, Reference Cohen, Eidem, Cetta, Fogel, Frommelt and Ganame295].
Neoaortic root dilatation and neoaortic valve regurgitation are the most frequently observed postoperative sequelae during long-term follow-up. Both findings can be detected and quantified using transthoracic echocardiography. However, exact quantification of the diameter of the aortic root and of regurgitant flow through the aortic valve, as well as of size and function of the left ventricle, may require examination by cardiac MRI [Reference Fratz, Chung, Greil, Samyn, Taylor and Valsangiacomo Buechel296].
Obstructed coronary arteries occur in 5–7% of survivors and remain the most common cause of morbidity and mortality after the ASO procedure [Reference Legendre, Losay, Touchot-Kone, Serraf, Belli and Piot297, Reference Ou, Khraiche, Celermajer, Agnoletti, Le Quan Sang and Thalabard298]. Invasive X-ray coronary angiography is still considered the gold standard for evaluation of coronary patency or obstruction; however, modern ECG-triggered computed tomography (CT) angiography provides excellent spatial resolution, has been validated in large studies [Reference Cho, Chang, Shin, Sung and Lin299] and can be used for morphological assessment in young adults after an ASO [Reference Ou, Celermajer, Marini, Agnoletti, Vouhé and Brunelle300].
In children, the use of coronary CT angiography is limited by the burden of radiation and the fast heart rate. Cardiac MRI is not validated for final evaluation of the coronary lumen but provides crucial functional information about myocardial perfusion (from first-pass perfusion images) and about the presence of myocardial scars (from late-enhancement images). In patients undergoing an ASO, cardiac MRI first pass has been shown to have slightly better diagnostic performance than positron emission tomography scans [Reference Tobler, Motwani, Wald, Roche, Verocai and Iwanochko301]. This technique has also been validated in children [Reference Buechel, Balmer, Bauersfeld, Kellenberger and Schwitter302, Reference Taylor, Dymarkowski, Hamaekers, Razavi, Gewillig and Mertens303]. Thus, the current recommendation is to perform noninvasive screening for myocardial ischaemia, with exercise testing (in older children and adults) and 24-h ECG (in younger children), in combination with cardiac MRI.
7.3 Arrhythmias
Postoperative early and late arrhythmias may have various causes but can also be related to subacute or acute myocardial ischaemia due to impaired coronary artery blood flow. Patients with certain unusual coronary patterns (including those with an intramural or single coronary artery) have a significantly increased mortality risk [Reference Prêtre, Tamisier, Bonhoeffer, Mauriat, Pouard and Sidi139, Reference Pasquali, Hasselblad, Li, Kong and Sanders143, Reference Planche, Bruniaux, Lacour-Gayet, Kachaner, Binet and Sidi154, Reference Day, Laks and Drinkwater304, Reference Yamaguchi, Hosokawa, Imai, Kurosawa, Yasui and Yagihara305]. Arrhythmia problems encountered include:
-
1. Supraventricular tachycardia and sinus node dysfunction are rarely seen after an ASO but are common after senning or mustard procedures [Reference el-Said, Rosenberg, Mullins, Hallman, Cooley and McNamara306–Reference Martin, Smith, Hernandez and Weldon308].
-
2. Sudden death: of all forms of congenital cardiac lesions, in the adult population, TGA is associated with one of the highest risks of sudden cardiac death [Reference Gelatt, Hamilton, McCrindle, Connelly, Davis and Harris269]. For children, adverse prognostic indicators are right ventricular dysfunction and QRS prolongation [Reference Wong, Finucane, Kerr, O’donnell, West and Gentles146, Reference Hayashi, Kurosaki, Echigo, Kado, Fukushima and Yokota309, Reference Yamazaki, Yamamoto, Sakamoto, Ishihara, Iwata and Matsumura310].
Thus, unlike in TGA patients with atrial switch repairs, arrhythmias are uncommon after an ASO (≤5%), possibly due to limited atrial manipulation [Reference Rhodes, Wernovsky, Keane, Mayer, Shuren and Dindy311]. Most patients who have had the ASO have, in fact, preserved sinus node function at long-term follow-up [Reference Rhodes, Wernovsky, Keane, Mayer, Shuren and Dindy311]. However, ASO patients are not completely free from arrhythmic complications. Longer aortic cross-clamp time and early age at time of operation have been identified as risk factors for rhythm disturbances among paediatric cardiac patients [Reference Delaney, Moltedo, Dziura, Kopf and Snyder312].

ASO: arterial switch operation; CMR: cardiovascular magnetic resonance; ECG: electrocardiogram; TGA: transposition of the great arteries.
a Class of recommendation.
b Level of evidence.
c References.
Recommendations for cardiology follow-up and arrhythmias
8. Reoperations and Interventions for Management of Late Complications
8.1 Introduction
With the transition from atrial to arterial repair of TGA, complications requiring reoperation have shifted mainly from the inflow to the outflow of the ventricles [Reference Losay, Touchot, Serraf, Litvinova, Lambert and Piot313, Reference Horer, Schreiber, Cleuziou, Vogt, Prodan and Busch314]. Most reoperations are performed during the first year after an ASO. This initial hazard phase is followed by a period of very low risk for reoperation, with a slightly ascending late hazard phase due to the increasing need for pulmonary artery and neoaortic valve surgery [Reference Losay, Touchot, Serraf, Litvinova, Lambert and Piot313]. Most (>80%) late reoperations are performed within the first 10 years after an ASO. Survival and functional outcome seem unaffected by the need for reintervention [Reference Angeli, Raisky, Bonnet, Sidi and Vouhé293]. Freedom from reoperation after a neonatal ASO is about 80% at 20 years [Reference Fricke, D’Udekem, Richardson, Thuys, Dronavalli and Ramsay3, Reference Lim, Kim, Lee and Kim5, Reference Oda, Nakano, Sugiura, Fusazaki, Ishikawa and Kado6, Reference Khairy, Clair, Fernandes, Blume, Powell and Newburger141, Reference Raju, Burkhart, Durham, Eidem, Phillips and Li169, Reference Lalezari, Bruggemans, Blom and Hazekamp171, Reference Horer, Schreiber, Cleuziou, Vogt, Prodan and Busch314]. The overall catheter reintervention rate is approximately 18% at 10 years and 25% at 25 years post-ASO [Reference Khairy, Clair, Fernandes, Blume, Powell and Newburger141, Reference Rodriguez Puras, Cabeza-Letran, Romero-Vazquianez, Santos de Soto, Hosseinpour and Gil Fournier315]. Most reinterventions are carried out during childhood [Reference Kempny, Wustmann, Borgia, Dimopoulos, Uebing and Li4, Reference Khairy, Clair, Fernandes, Blume, Powell and Newburger141, Reference Tobler, Williams, Jegatheeswaran, Van Arsdell, McCrindle and Greutmann145]. The risk for reintervention in adulthood is significantly higher for those having already had one reintervention during childhood [Reference Tobler, Williams, Jegatheeswaran, Van Arsdell, McCrindle and Greutmann145].
8.2 Reoperations for neopulmonary outflow tract lesions
8.2.1 Relief of neopulmonary outflow tract obstruction
8.2.1.1 Neopulmonary outflow tract obstruction: prevalence and incidence
Neopulmonary outflow tract obstruction is the most frequent cause for reoperation [Reference Haas, Wottke, Poppert and Meisner316–Reference Nogi, McCrindle, Boutin, Williams, Freedom and Benson318]. Obstruction may occur at multiple levels following an ASO but most frequently involves the pulmonary arteries, with a reported incidence of 1–42% [Reference Prêtre, Tamisier, Bonhoeffer, Mauriat, Pouard and Sidi139, Reference Ullmann, Gorenflo, Bolenz, Sebening, Goetze and Arnold166, Reference Hutter, Kreb, Mantel, Hitchcock, Meijboom and Bennink167, Reference Rudra, Mavroudis, Backer, Kaushal, Russell and Stewart173, Reference Angeli, Raisky, Bonnet, Sidi and Vouhé293]. Neopulmonary valve stenosis tends to develop primarily during the first year after repair [Reference Wernovsky, Mayer, Jonas, Hanley, Blackstone and Kirklin319], but there is a persistent constant low risk thereafter [Reference Losay, Touchot, Serraf, Litvinova, Lambert and Piot313, Reference Wernovsky, Mayer, Jonas, Hanley, Blackstone and Kirklin319].
8.2.1.2 Neopulmonary outflow tract obstruction: patient-related risk factors
-
1. Younger age at operation and lower birth weight [Reference Wernovsky, Mayer, Jonas, Hanley, Blackstone and Kirklin319–Reference Vargo, Mavroudis, Stewart and Backer321].
-
2. Coronary artery pattern (e.g. left coronary artery arising from the right-facing sinus) [Reference Wernovsky, Mayer, Jonas, Hanley, Blackstone and Kirklin319–Reference Vargo, Mavroudis, Stewart and Backer321].
-
3. Size mismatch between the great arteries and a smaller native aortic annulus [Reference Bove, De Meulder, Vandenplas, De Groote, Panzer and Suys322].
-
4. Side-by-side relationship of the great arteries [Reference Williams, Quaegebeur, Kirklin and Blackstone320].
-
5. Coexisting aortic coarctation (with a small native aortic annulus) [Reference Williams, Quaegebeur, Kirklin and Blackstone320].
-
6. Rapid somatic growth [Reference Angeli, Raisky, Bonnet, Sidi and Vouhé293, Reference Prifti, Crucean, Bonacchi, Bernabei, Murzi and Luisi323].
-
7. Remnant ductal tissue causing left pulmonary artery stenosis [Reference Lupinetti, Bove, Minich, Snider, Callow and Meliones324, Reference Paillole, Sidi, Kachaner, Planche, Belot and Villain325].
-
8. Earlier institutional experience [Reference Williams, Quaegebeur, Kirklin and Blackstone320].
8.2.1.3 Neopulmonary outflow tract obstruction: surgery-related risk factors
-
1. Diffuse hypoplasia of the main pulmonary artery may be secondary to several mechanisms:
-
1. Inadequate mobilization of the branch pulmonary arteries [Reference Wernovsky, Hougen, Walsh, Sholler, Colan and Sanders326, Reference Spiegelenberg, Hutter, van de Wal, Hitchcock, Meijboom and Harinck327].
-
2. Technique used for reconstruction of the proximal pulmonary artery [Reference Lupinetti, Bove, Minich, Snider, Callow and Meliones324, Reference Paillole, Sidi, Kachaner, Planche, Belot and Villain325, Reference Zeevi, Keane, Perry and Lock328]. Distortion and stretching of the main and branch pulmonary arteries occur as a result of the Lecompte manoeuvre [Reference Kuroczynski, Kampmann, Choi, Hilker, Wippermann and David329].
-
3. Posterior compression of the neopulmonary valve and main pulmonary artery by the neoaorta, also as a result of the Lecompte manoeuvre [Reference Massin, Nitsch, Dabritz, Seghaye, Messmer and von Bernuth330].
-
4. Pulmonary artery banding causing supravalvular stenosis [Reference Nakanishi, Momoi, Satoh, Yamada, Terada and Nakazawa331, Reference Serraf, Bruniaux, Lacour-Gayet, Sidi, Kachaner and Bouchart332].
-
5. Growth failure of the valve annulus [Reference Nogi, McCrindle, Boutin, Williams, Freedom and Benson318, Reference Nakanishi, Momoi, Satoh, Yamada, Terada and Nakazawa331].
-
6. Use of prosthetic material in reconstruction of the neopulmonary sinuses [Reference Williams, Quaegebeur, Kirklin and Blackstone320].
-
2. Discrete, circumferential narrowing at the suture line, leading to inadequate growth of the pulmonary anastomotic site resulting in a more discrete type of stenosis [Reference Angeli, Raisky, Bonnet, Sidi and Vouhé293, Reference Wernovsky, Mayer, Jonas, Hanley, Blackstone and Kirklin319].
8.2.1.4 Neopulmonary outflow tract obstruction: indications for treatment.
Reoperation or intervention is indicated when a gradient >50 mmHg at any level in the neopulmonary outflow tract is detected on routine echo Doppler evaluation [Reference Angeli, Raisky, Bonnet, Sidi and Vouhé293]. Supravalvular pulmonary stenosis should be relieved early because it may be associated with growth failure of the valve annulus [Reference Raja, Shauq and Kaarne333, Reference Santoro, Di Carlo, Formigari and Ballerini334] and may induce asymmetrical distribution of pulmonary flow [Reference Massin, Nitsch, Dabritz, Seghaye, Messmer and von Bernuth330].
8.2.1.5 Neopulmonary outflow tract obstruction: intervention.
Some patients who have had an ASO may need a reintervention to alleviate pulmonary branch artery stenosis. Although the arterial diameter could be increased in some cases using balloon dilation, the improvements did not last, and a high recurrence rate was observed. These unfavourable results led to the use of stents, which were significantly more effective [Reference Formigari, Santoro, Guccione, Giamberti, Pasquini and Grigioni335]. The risks of early dissection and vessel rupture are known and manageable. More recently, a potentially more serious complication has been described—creation of an aortopulmonary window. This complication cannot be avoided entirely because overdilation is necessary to achieve long-term success, but cardiologists should be aware of it [Reference Tzifa, Papagiannis and Qureshi336, Reference Torres, Sanders, Vincent, El-Said, Leahy and Padera337].

RVOT: right ventricular outflow tract.
a Class of recommendation.
b Level of evidence.
c References.
Recommendations for late neopulmonary outflow tract obstruction
8.2.2 Treatment of neopulmonary valve regurgitation
8.2.2.1 Neopulmonary valve regurgitation: prevalence and incidence.
Neopulmonary valve regurgitation occurs after an ASO with an incidence varying from 9% to 80% [Reference Gleason, Chin, Andrews, Barber, Helton and Murphy338, Reference Martin, Snider, Bove, Serwer, Rosenthal and Peters339]. At least moderate neopulmonary valve regurgitation was present in 6.6% of the cases in the series of Khairy et al. [Reference Khairy, Clair, Fernandes, Blume, Powell and Newburger141].
8.2.2.2 Neopulmonary valve regurgitation: potential risk factors
-
1. Valve distortion secondary to anterior displacement of the neopulmonary arterial root by a redundant posterior neoaorta.
-
2. Valve misalignment during patch reconstruction of the neopulmonary artery root.
-
3. Loss of the sinotubular junction of the neopulmonary artery root following single-patch reconstruction of the coronary defects.
-
4. Partial commissural detachment to harvest a paracommissural or intramural coronary artery.
8.2.2.3 Neopulmonary valve regurgitation: indications for treatment
Reported cases of treated neopulmonary valve regurgitation are sporadic and usually embedded in large series of late results from ASOs [Reference Khairy, Clair, Fernandes, Blume, Powell and Newburger141, Reference Hovels-Gurich, Seghaye, Ma, Miskova, Minkenberg and Messmer340]. An increasing number of such cases are anticipated as the ASO population gets older. Indications for neopulmonary valve surgery probably replicate those for any long-standing pulmonary-valve regurgitation affecting right ventricular function [Reference Geva341]. Surgical pulmonary valvuloplasty is attempted with increasing frequency [Reference Khairy, Clair, Fernandes, Blume, Powell and Newburger141]. However, pulmonary valve replacement still is often required with a preference for biological prostheses to allow later transcatheter implantation of other prostheses [Reference Eicken, Ewert, Hager, Peters, Fratz and Kuehne342, Reference Thanopoulos, Giannakoulas and Arampatzis343], although some concern has been raised about potential coronary compression [Reference Biermann, Schonebeck, Rebel, Weil and Dodge-Khatami344].

a Class of recommendation.
b Level of evidence.
c References.
Recommendations for reoperations for late neopulmonary valve regurgitation
8.3 Reoperations for left ventricular outflow tract lesions
8.3.1 Treatment of neoaortic valve regurgitation
8.3.1.1 Neoaortic regurgitation and root dilatation: prevalence and incidence
After an ASO, the native pulmonary valve faces the high-pressure systemic circulation. It is still unclear whether it can maintain long-term competence [Reference Angeli, Raisky, Bonnet, Sidi and Vouhé293, Reference Vargo, Mavroudis, Stewart and Backer321]. Neoaortic regurgitation is the second most common indication for reoperation after an ASO [Reference Raju, Burkhart, Durham, Eidem, Phillips and Li169, Reference Haas, Wottke, Poppert and Meisner316], needed in 5–22% of cases [Reference Delmo Walter, Huebler, Alexi-Meshkishvili, Sill, Berger and Hetzer345–Reference Schwartz, Gauvreau, del Nido, Mayer and Colan348]. It was more frequent in the early years of ASOs, occurring in up to 55% of patients undergoing a two-stage operation [Reference Martin, Ettedgui, Qureshi, Gibbs, Baker and Radley-Smith349, Reference Lange, Cleuziou, Horer, Holper, Vogt and Tassani-Prell350]. In the current era of neonatal ASOs, moderate-to-severe neoaortic regurgitation is less frequent (<7%) [Reference Marino, Wernovsky, McElhinney, Jawad, Kreb and Mantel346–Reference Schwartz, Gauvreau, del Nido, Mayer and Colan348]; the need for reoperation at 15 years is ≤3% [Reference Haas, Wottke, Poppert and Meisner316]. Of concern, however, is the high prevalence of trivial-to-mild neoaortic regurgitation and neoaortic root dilation, with some evidence of progression [Reference Haas, Wottke, Poppert and Meisner316, Reference Marino, Wernovsky, McElhinney, Jawad, Kreb and Mantel346, Reference Lange, Cleuziou, Horer, Holper, Vogt and Tassani-Prell350–Reference Hwang, Kim, Kwak, Lee, Kim and Rho352]. Aortic root dilation usually stabilizes over time [Reference Schwartz, Gauvreau, del Nido, Mayer and Colan348, Reference Hutter, Thomeer, Jansen, Hitchcock, Faber and Meijboom353]. Fortunately, neoaortic valve intervention is associated with good outcomes [Reference Mavroudis, Stewart, Backer, Rudra, Vargo and Jacobs354, Reference Losay, Touchot, Capderou, Piot, Belli and Planche355].
8.3.1.2 Neoaortic regurgitation and root dilatation: patient-related risk factors
-
1. Intrinsic weakness of native pulmonary artery and valve tissue in facing systemic pressure [Reference Raju, Burkhart, Durham, Eidem, Phillips and Li169, Reference del Nido and Schwartz356–Reference Niwa, Perloff, Bhuta, Laks, Laks, Drinkwater and Child359], although others have refuted this hypothesis [Reference Bottio, Thiene, Tarzia, Rizzoli and Gerosa360].
-
2. Previous pulmonary banding, distorting the native pulmonary artery root [Reference Vargo, Mavroudis, Stewart and Backer321, Reference Marino, Wernovsky, McElhinney, Jawad, Kreb and Mantel346, Reference Losay, Touchot, Capderou, Piot, Belli and Planche355].
-
3. The diameter of the neoaortic root appears positively related with mild-to-moderate neoaortic regurgitation [Reference Bove, De Meulder, Vandenplas, De Groote, Panzer and Suys322, Reference Co-Vu, Ginde, Bartz, Frommelt, Tweddell and Earing361, Reference Lalezari, Mahtab, Bartelings, Wisse, Hazekamp and Gittenberger-de Groot362]. Freedom from neoaortic root dilation at 1, 5, 10 and 15 years after an ASO is 84%, 67%, 47% and 32%, respectively [Reference Co-Vu, Ginde, Bartz, Frommelt, Tweddell and Earing361]. Others, however, observed that rapid dilation of the neoaorta is limited to the first year of life [Reference Hutter, Thomeer, Jansen, Hitchcock, Faber and Meijboom353].
-
4. Non-facing commissures [Reference Michalak, Moll, Moll, Dryzek, Moszura and Kopala363].
-
5. Abnormal coronary artery anatomy [Reference Schwartz, Gauvreau, del Nido, Mayer and Colan348, Reference Formigari, Toscano, Giardini, Gargiulo, Di Donato and Picchio364].
-
6. Preoperative pulmonary valve regurgitation [Reference Hwang, Kim, Kwak, Lee, Kim and Rho352].
-
7. Acute angulation of the aortic arch following posterior translocation of the ascending aorta [Reference Agnoletti, Ou, Celermajer, Boudjemline, Marini and Bonnet365].
-
8. Impaired growth of the neoaorta [Reference Formigari, Toscano, Giardini, Gargiulo, Di Donato and Picchio364].
8.3.1.3 Neoaortic regurgitation and root dilatation: surgery-related risk factors
-
1. The trap-door technique may cause distortion of the geometry of the sinotubular junction [Reference Formigari, Toscano, Giardini, Gargiulo, Di Donato and Picchio364], although others have disputed this suggestion [Reference Jhang, Shin, Park, Yun, Kim and Ko366]. Very large buttons for translocation of the coronary arteries can also distort.
-
2. Aortic transection with disruption of vasa vasorum may alter the structure of the neoaorta [Reference McMahon, Ravekes, Smith, Denfield, Pignatelli and Altman347, Reference Murakami, Nakazawa, Momma and Imai367].
8.3.1.4 Neoaortic root dilation and neoaortic valve regurgitation: treatment
Surgery on the neoaortic root or valve is rare after an ASO and is needed mainly in children aged >1 year at the time of the ASO, particularly those undergoing conversion of an atrial switch [Reference Fricke, Brizard, D’Udekem and Konstantinov368]. The indications for aortic valve surgery are the same as those for adults with aortic regurgitation [Reference Marino, Bridges and Paridon369]. The indications and timing for repair of neoaortic root dilation are unclear [Reference Co-Vu, Ginde, Bartz, Frommelt, Tweddell and Earing361]. Nevertheless, elective neoaortic root operations after an ASO have been reported for patients with neoaortic root z-scores of ≥3 [Reference Schwartz, Gauvreau, del Nido, Mayer and Colan348]. If neoaortic regurgitation occurs early, the patient should be closely monitored [Reference Vargo, Mavroudis, Stewart and Backer321]. Importantly, the operation should be advised before the development of severe aortic regurgitation [Reference Raju, Burkhart, Durham, Eidem, Phillips and Li169]. Surgical options adopted for neoaortic valve regurgitation with or without neoaortic root dilation include:
-
1. Valve-sparing aortic root repair, preventing the need for anticoagulation [Reference Mavroudis, Stewart, Backer, Rudra, Vargo and Jacobs354, Reference Imamura, Drummond-Webb, McCarthy and Mee370].
-
2. Prosthetic valve replacement [Reference Oda, Nakano, Sugiura, Fusazaki, Ishikawa and Kado6, Reference Mavroudis, Stewart, Backer, Rudra, Vargo and Jacobs354, Reference Losay, Touchot, Capderou, Piot, Belli and Planche355, Reference Lalezari, Mahtab, Bartelings, Wisse, Hazekamp and Gittenberger-de Groot362, Reference Fricke, Brizard, D’Udekem and Konstantinov368, Reference Alexi-Meskishvili, Photiadis and Nurnberg371].
-
3. Bentall operation [Reference Oda, Nakano, Sugiura, Fusazaki, Ishikawa and Kado6, Reference Fricke, Brizard, D’Udekem and Konstantinov368].
-
4. Switch-back operation (Ross after an ASO) [Reference Hazekamp, Schoof, Suys, Hutter, Meijboom and Ottenkamp372, Reference Vicente, Ferreira, Klamt, Manso, Filho and Carlotti373].

a Class of recommendation.
b Level of evidence.
c References.
Recommendations for reoperations for late neoaortic valve regurgitation
8.3.2 Relief of neoaortic outflow tract obstruction
Left-sided obstruction needing reoperation after an ASO is considerably less frequent than neopulmonary outflow tract obstruction, with freedom from reinterventions of up to 99% at 10 years after an ASO [Reference Williams, Quaegebeur, Kirklin and Blackstone320, Reference Hourihan, Colan, Wernovsky, Maheswari, Mayer and Sanders374, Reference Leobon, Belli, Ly, Kortas, Le Bret and Sigal-Cinqualbre375]. However, at least mild late neoaortic stenosis was found in 3.2% of ASO patients [Reference Khairy, Clair, Fernandes, Blume, Powell and Newburger141]. Reintervention for left-sided obstruction after an ASO may consist of direct relief of the residual LVOT obstruction or of patch angioplasty of the ascending aorta [Reference Williams, Quaegebeur, Kirklin and Blackstone320]. Neoaortic valvular stenosis with a small annulus after an ASO can be managed using the Konno procedure [Reference Leobon, Belli, Ly, Kortas, Le Bret and Sigal-Cinqualbre375, Reference Kosaka, Kurosawa and Nagatsu376].
8.4 Reoperations for coronary lesions
Coronary obstructions may develop even after a successful ASO and can lead to coronary events [Reference Tamisier, Ouaknine, Pouard, Mauriat, Lefebvre and Sidi377]. Coronary events show a bimodal pattern with a rapidly declining early phase and a slowly increasing late phase [Reference Legendre, Losay, Touchot-Kone, Serraf, Belli and Piot297]. The left main coronary artery is more frequently affected [Reference Oda, Nakano, Sugiura, Fusazaki, Ishikawa and Kado6, Reference Ou, Khraiche, Celermajer, Agnoletti, Le Quan Sang and Thalabard298].
8.4.1 Mechanisms for long-term declining coronary function
Progressive proximal eccentric intimal thickening occurs in up to 89% of the coronary arteries after an ASO [Reference Pedra, Pedra, Abizaid, Braga, Staico and Arrieta378]. The complexity of coronary patterns and the modality of coronary transfer could prompt flow abnormalities, leading to increased shear stress and progressive fibrocellular intimal thickening [Reference Angeli, Raisky, Bonnet, Sidi and Vouhé293, Reference Legendre, Losay, Touchot-Kone, Serraf, Belli and Piot297, Reference Pedra, Pedra, Abizaid, Braga, Staico and Arrieta378, Reference Bartoloni, Bianca, Patane and Mignosa379]. Other suggested causes of progressive coronary occlusion include ostial fibrosis at the suture line, kinking or stretching with reactive injury from surgical manipulation [Reference Vargo, Mavroudis, Stewart and Backer321, Reference Bonnet, Bonhoeffer, Piechaud, Aggoun, Sidi and Planche380, Reference Tanel, Wernovsky, Landzberg, Perry and Burke381]. A hypothetical compensatory mechanism is the greater capacity of collateralization, neovascularization and cellular proliferation of infants compared with adults [Reference Tanel, Wernovsky, Landzberg, Perry and Burke381]. In reality, however, infant coronary collateralization is unpredictable, and survival relies mostly on the number of arterial segments remaining patent [Reference Mavroudis, Stewart, Backer, Rudra, Vargo and Jacobs354]. As the ASO population approaches adulthood, superimposed variations in coronary artery anatomy will conceivably increase the risk factors for atherosclerotic disease and ischaemic events [Reference Vargo, Mavroudis, Stewart and Backer321]. Therefore, these patients should undergo life-long close monitoring of myocardial perfusion [Reference Angeli, Formigari, Pace Napoleone, Oppido, Ragni and Picchio382].
8.4.2 Coronary lesions: prevalence, incidence and diagnosis
The known incidence of late coronary stenosis requiring reintervention varies from <3% to 10% [Reference Khairy, Clair, Fernandes, Blume, Powell and Newburger141, Reference Pasquali, Hasselblad, Li, Kong and Sanders143, Reference Legendre, Losay, Touchot-Kone, Serraf, Belli and Piot297, Reference Losay, Touchot, Serraf, Litvinova, Lambert and Piot313, Reference Raisky, Bergoend, Agnoletti, Ou, Bonnet and Sidi383], and silent coronary obstructive lesions have a prevalence of 6–8% [Reference Legendre, Losay, Touchot-Kone, Serraf, Belli and Piot297, Reference Bonnet, Bonhoeffer, Piechaud, Aggoun, Sidi and Planche380]. Some patients are symptom-free, without diagnostic evidence of myocardial ischaemia, until they experience sudden cardiac events [Reference Angeli, Raisky, Bonnet, Sidi and Vouhé293, Reference Legendre, Losay, Touchot-Kone, Serraf, Belli and Piot297, Reference Vargo, Mavroudis, Stewart and Backer321, Reference Bonhoeffer, Bonnet, Piechaud, Stumper, Aggoun and Villain384, Reference Hutter, Bennink, Ay, Raes, Hitchcock and Meijboom385]. Coronary lesions were detected after a mean interval of 33 months. Because the lesions are usually progressive, coronary evaluation should be repeated regularly during late follow-up [Reference Angeli, Raisky, Bonnet, Sidi and Vouhé293].
Indications for selective coronary angiography or multislice CT angiography after an ASO include (i) the presence of electrocardiographic or echocardiographic signs suggestive of myocardial ischaemia at any time after the operation; (ii) the presence of unusual coronary patterns (e.g. coronary arteries coursing between the great arteries) or intraoperative difficulties in coronary transfer; and (iii) the use of a single-orifice technique for coronary reimplantation.
8.4.3 Coronary lesions: patient-related risk factors
-
1. Complex coronary anatomy. Early coronary lesions and coronary reoperation rates are more frequent in patients with complex or unusual coronary patterns [Reference Metton, Calvaruso, Gaudin, Mussa, Raisky and Bonnet142, Reference Rudra, Mavroudis, Backer, Kaushal, Russell and Stewart173, Reference Legendre, Losay, Touchot-Kone, Serraf, Belli and Piot297, Reference Vargo, Mavroudis, Stewart and Backer321, Reference Tamisier, Ouaknine, Pouard, Mauriat, Lefebvre and Sidi377, Reference Angeli, Formigari, Pace Napoleone, Oppido, Ragni and Picchio382]. Intramural origin or single-ostium looping was found to increase the risk for morbidity and mortality [Reference Pasquali, Hasselblad, Li, Kong and Sanders143]. Others did not find this correlation [Reference Thrupp, Gentles, Kerr and Finucane144, Reference Hutter, Bennink, Ay, Raes, Hitchcock and Meijboom385].
-
2. Bicuspid neoaortic valve. This condition might cause commissural malalignment and neoaortic root dilation; both conditions increase the risk for suboptimal coronary transfer [Reference Angeli, Gerelli, Beyler, Lamerain, Rochas and Bonnet386].
-
3. Relative position of the great arteries. Peculiar relationships between the reimplanted coronary arteries and the adjacent great arteries might lead to coronary compression [Reference Legendre, Losay, Touchot-Kone, Serraf, Belli and Piot297, Reference Bonnet, Bonhoeffer, Piechaud, Aggoun, Sidi and Planche380].
8.4.4 Coronary lesions: surgery-related risk factors
Technical details may impact on the need for reintervention after an ASO, and late coronary lesions may occur in all coronary patterns, even after the most straightforward initial operation [Reference Angeli, Raisky, Bonnet, Sidi and Vouhé293].
Mechanical reasons for coronary obstruction include proximal coronary artery compression, kinking and stretching. Various technical factors have been suspected: inadequate coronary transfer, type of coronary artery button technique, excessive use of fibrin glue and abnormal early fibrosis [Reference Rudra, Mavroudis, Backer, Kaushal, Russell and Stewart173, Reference Angeli, Raisky, Bonnet, Sidi and Vouhé293, Reference Legendre, Losay, Touchot-Kone, Serraf, Belli and Piot297, Reference Ou, Khraiche, Celermajer, Agnoletti, Le Quan Sang and Thalabard298, Reference Mavroudis, Stewart, Backer, Rudra, Vargo and Jacobs354].
8.4.5 Residual/recurrent coronary lesions: indications and management
Acute coronary insufficiency at the time of an ASO must be immediately addressed by revision of the anastomosis or by bypass grafting.
Reoperation in the event of late coronary insufficiency is indicated when myocardial ischaemia is demonstrated at myocardial imaging.
Different approaches have been used to manage post-ASO coronary lesions.
8.4.5.1 Surgical approaches
-
1. Coronary (ostial) patch angioplasty, using saphenous vein patch [Reference Angeli, Raisky, Bonnet, Sidi and Vouhé293, Reference Bonnet, Bonhoeffer, Sidi, Kachaner, Acar and Villain387, Reference Raanani, Kogan, Shapira, Sagie, Kornowsky and Vidne388], autologous or heterologous pericardial patch [Reference Vargo, Mavroudis, Stewart and Backer321, Reference Raisky, Bergoend, Agnoletti, Ou, Bonnet and Sidi383, Reference Bonnet, Bonhoeffer, Sidi, Kachaner, Acar and Villain387–Reference Prifti, Bonacchi, Luisi and Vanini391] or the proximal segment of the right internal thoracic artery [Reference Jonsson, Jensen, Olsson, Holm and Liska392]. Adequate enlargement of a proximal coronary obstruction is achieved using a patch from the aorta, across the stenotic lesion, down to a normal coronary artery, restoring normal coronary perfusion [Reference Bonnet, Bonhoeffer, Sidi, Kachaner, Acar and Villain387, Reference Prêtre and Turina390, Reference Dion, Verhelst, Matta, Rousseau, Goenen and Chalant393]. Surgical arterioplasty might be contraindicated only when the left main coronary artery bifurcation is involved in the stenotic process [Reference Bergoend, Raisky, Degandt, Tamisier, Sidi and Vouhé389]. Coronary ostial patch angioplasty can be performed with low operative risk and high patency rate [Reference Bonhoeffer, Bonnet, Piechaud, Stumper, Aggoun and Villain384]. However, its long-term prognosis remains unknown.
-
2. Internal mammary artery grafting is technically feasible in most children, with satisfactory patency rates [Reference Rudra, Mavroudis, Backer, Kaushal, Russell and Stewart173, Reference Angeli, Raisky, Bonnet, Sidi and Vouhé293, Reference Mavroudis, Stewart, Backer, Rudra, Vargo and Jacobs354, Reference Bergoend, Raisky, Degandt, Tamisier, Sidi and Vouhé389, Reference Mavroudis, Backer, Duffy, Pahl and Wax394, Reference Mavroudis, Backer, Muster, Pahl, Sanders and Zales395]. Internal mammary grafting has been suggested only for more distal lesions, long and complete occlusions of the main stem or residual obstruction after primary surgical arterioplasty [Reference Bergoend, Raisky, Degandt, Tamisier, Sidi and Vouhé389]. However, blood flow through a mammary bypass may be inadequate, particularly when a large myocardial area must be revascularized [Reference Mavroudis, Backer, Duffy, Pahl and Wax394, Reference Mavroudis, Backer, Muster, Pahl, Sanders and Zales395].
8.4.5.2 Percutaneous transluminal coronary angioplasty in infants and young children
This procedure includes the possibility of inserting a coronary stent, as a first-time intervention to alleviate coronary stenosis, or after a previous coronary patch angioplasty operation [Reference Angeli, Formigari, Pace Napoleone, Oppido, Ragni and Picchio382, Reference Kampmann, Kuroczynski, Trubel, Knuf, Schneider and Heinemann396–Reference El-Segaier, Lundin, Hochbergs, Jogi and Pesonen398]. In these rare patients a policy of routine angiographic evaluation of the coronary arteries within the first 2–3 years after repair is advisable [Reference Angeli, Formigari, Pace Napoleone, Oppido, Ragni and Picchio382].

ASO: arterial switch operation; CT: computed tomography; MRI: magnetic resonance imaging.
a Class of recommendation.
b Level of evidence.
c References.
Recommendations for reoperations for residual or recurrent coronary lesions
8.5 Other reoperations for late complications
8.5.1 Treatment of tracheobronchial compression
Tracheobronchial [Reference Robotin, Bruniaux, Serraf, Uva, Roussin and Lacour-Gayet402–Reference Worsey, Pham, Newman, Park and del Nido404] and oesophageal [Reference McElhinney, Reddy, Reddy, Higgins and Hanley405] compression by vascular structures (aorta or pulmonary arteries) may develop after an ASO and require surgical relief.
The mechanism of ‘left bronchial compression’ relates to the posterior displacement of the ascending aorta behind the left pulmonary artery with either impingement of this vessel upon the left main bronchus or compression of the bronchus between the ascending and descending aorta (pincer effect) [Reference Worsey, Pham, Newman, Park and del Nido404, Reference Gandhi, Pigula and Siewers406]. Tracheography is a useful diagnostic method in cases with airway obstruction [Reference Toker, Tireli, Bostanci, Ozcan and Dayioglu403, Reference Corno, Giamberti, Giannico, Marino, Rossi and Marcelletti407]. Post-ASO left bronchial compression may be prevented using high transection on the great arteries and the Lecompte manoeuvre, leaving the anastomotic region above the left main bronchus level [Reference Toker, Tireli, Bostanci, Ozcan and Dayioglu403]. Compression of the left main bronchus by the posteriorly displaced aorta can be approached by an aortopexy procedure, through a left thoracotomy. In cases of persistent symptoms, tracheobronchomalacia must be ruled out [Reference Robotin, Bruniaux, Serraf, Uva, Roussin and Lacour-Gayet402].
8.5.2 Treatment of persistent pulmonary hypertension
Pulmonary vascular disease and pulmonary hypertension in TGA IVS are rare but severe [Reference Kirklin, Blackstone, Tchervenkov and Castaneda138, Reference Haas, Wottke, Poppert and Meisner316, Reference Daebritz, Nollert, Sachweh, Engelhardt, von Bernuth and Messmer351, Reference Kumar, Taylor, Sandor and Patterson408] conditions that can occur even in patients undergoing an ASO before the age of 1 month [Reference Losay, Touchot, Serraf, Litvinova, Lambert and Piot313]. Medical therapy should first be provided [Reference Damen and Hitchcock409], but for the most resilient cases, surgical management may be attempted, using either blade atrial septostomy [Reference Kerstein, Levy, Hsu, Hordof, Gersony and Barst410] or a Potts anastomosis [Reference Angeli, Raisky, Bonnet, Sidi and Vouhé293, Reference Blanc, Vouhé and Bonnet411]. The goal of the latter procedure is to decrease right ventricular afterload, which improves right ventricular function and potentially prevents syncope and sudden death [Reference Blanc, Vouhé and Bonnet411].
8.5.3 Treatment of transposition of the great arteries with aortopulmonary collaterals
Aortopulmonary collaterals have long been known to coexist with TGA [Reference Aziz, Paul and Rowe412]. They present angiographically as enlarged bronchial arteries [Reference Wernovsky, Bridges, Mandell, Castaneda and Perry413]. Significant collateral flow may first become manifest during surgical correction, when significant left atrial or pulmonary artery return is noticed during CPB; intraoperative pulmonary haemorrhage has also been reported [Reference Golej, Trittenwein, Marx and Schlemmer414]. Coil embolization can be carried out either before [Reference Jowett, Derrick, Tsang and Marek415] or after [Reference Wernovsky, Bridges, Mandell, Castaneda and Perry413–Reference Irving and Chaudhari416] an ASO, with complete occlusion of the vessels and no significant complications [Reference Wernovsky, Bridges, Mandell, Castaneda and Perry413, Reference Golej, Trittenwein, Marx and Schlemmer414, Reference Irving and Chaudhari416, Reference Santoro, Carrozza, Russo and Calabro417].
8.5.4 Treatment of residual mitral regurgitation
Mitral valve reoperations are rarely necessary for ischaemic mitral regurgitation or residual mitral valve malformation (mitral cleft) [Reference Raju, Burkhart, Durham, Eidem, Phillips and Li169, Reference Angeli, Raisky, Bonnet, Sidi and Vouhé293, Reference Losay, Touchot, Serraf, Litvinova, Lambert and Piot313].
Conflict of interest: none declared.






















