INTRODUCTION
It is estimated that about 390 million infections of dengue (DEN) occur every year worldwide, of which about 100 million have a clinical manifestation [Reference Bhatt1] making DEN the most important vector-borne viral disease. Over 100 countries in tropical and subtropical regions are endemic for DEN [2], with more than a billion people living in South East Asia at risk of DEN [Reference Saukari3]. With about 34% of the total estimated cases throughout the world, India is known to be hyperendemic for DEN [Reference Bhatt1]. Dengue virus (DENV), the causative agent belongs to the genus Flavivirus, family Flaviviridae. DENV has four serotypes that are antigenically related [Reference Lindenbach and Rice4]. An infection with any of the serotypes can result in a mild form of disease such as dengue fever (DF) that may lead to dengue haemorrhagic fever (DHF) and dengue shock syndrome (DSS) [Reference Gubler5]. A secondary infection by a heterologous serotype is a risk factor for DHF and DSS [Reference Halstead6]. The virus is transmitted by the Aedes species of mosquitoes and DEN incidence is known to be directly related to vector density [7]. High density of vector mosquitoes, favourable environmental conditions and movement of human populations are some of the factors responsible for outbreaks of DEN [8]. In the absence of any vaccine, vector control measures are the best way to avoid outbreaks.
All four serotypes with different genotypes are known to be circulating in India. In central India, DENV-3 was detected in 1966 from Jabalpur [Reference Rodrigues9], and in 1992 DENV-2 was detected in a focal outbreak from Chirimiri town [Reference Mahadev10]. Since 2007, national vector-borne disease control programme (NVBDCP) surveillance has reported sporadic cases of DEN from this part of the country [7]. Serotype detection, which is important in determining the virulence and severity of an outbreak, has not been documented during the last two decades in central India.
Based on media reports in August 2012 in Korea district, Chhattisgarh (CG), regarding febrile illness from Churcha colliery and high DEN positivity in referred cases from the villages of Narsinghpur district, Madhya Pradesh (MP), in November 2012 we conducted clinical and entomological surveys in the affected areas and employed serological and molecular tests on the samples collected in order to establish the aetiology of these outbreaks.
METHODS
Study areas and populations
Churcha colliery is located about 10 km from Korea district (between latitude 23° 02′ 42″ to 23° 44′ 46″ N, and longitude 81° 46′ 42″ to 82° 33′ 43″ E) in CG [11] (Fig. 1). The area has sprawling coal-mining tunnels surrounded by green forest and the mountains of Vindhaya. The average altitude is about 700 m above sea level with an annual rainfall of ~1410·9 mm. The average temperature is around 25 ± 2°C with relative humidity of 60–80% during August [11]. Churcha colliery is inhabited mainly by the employees of South East Colliery Limited (SECL) with their families, who come from all over the country and are of different socioeconomic backgrounds. About 6000 pucca houses are distributed in 16 wards. It is a semi-urban setting with a population of about 30 000. Following reports in the local print media regarding deaths due to febrile illness from this area in August 2012, investigations were conducted. Narsighpur district is located in the state of MP (between latitude 22° 55′ and 23° 15′ N, and longitude 78° 38′ and 79° 38′ E) (Fig. 1). DEN infection was detected in high proportions in samples referred to the Virology Diagnostic Laboratory, Jabalpur during November 2012 from two villages, Manesur and Imaliya, about 20 and 30 km, respectively, from Narsighpur district. The temperature averages around 18 ± 2°C during November. Manesur village has about 300 households with a population of 1800, while Imaliya village has 400 households with an approximate population of 1700.

Fig. 1. (a) Map of India showing (b) Madhya Pradesh and Chhattisgarh. (c) The outbreak districts of Korea and Narsinghpur are indicated by grey shading (map not to scale).
Clinical investigations
Health camps were set up in affected areas and blood samples (2 ml) were collected from patients showing signs and symptoms of DEN according to WHO guidelines [12] following clinical examination. Serum/plasma was separated by briefly centrifuging the blood at 4°C and transporting it to the virology laboratory under cold conditions.
Entomological surveillance
House-to-house surveys to collect adult and larvae of mosquitoes and to record their breeding sites were conducted following NVBDCP guidelines. Mosquito control measures such as fogging of insecticides, larvicidal treatment and destruction of larval habitats was initiated as recommended by NVBDCP [13]. Mosquito larvae were collected and reared in the laboratory. The adults were identified using a standard key [Reference Barnard14] and stored at −70°C in pools (10/pool) for viral detection.
Laboratory testing
The samples were screened in the field using rapid diagnostic kits for DEN IgM and NS1 protein (J. Mitra & Co., India) according to the manufacturer's instructions. In the laboratory samples collected after 5 days of onset of fever were tested by a NVBDCP-recommended DEN IgM ELISA kit manufactured by the National Institute of Virology (NIV), India following the kit's protocol. IgG for DEN was detected using DEN IgG Capture ELISA (Inverness Medical, Australia). The samples found negative for DEN IgM were also tested for Chikungunya (CHIK) IgM using the CHIK IgM ELISA kit manufactured by the NIV [Reference Yergolkar15].
For molecular diagnosis, the serum samples collected during the acute phase of illness were subjected to nested reverse transcriptase–polymerase chain reaction (nRT–PCR) as described by Lanciotti et al. [Reference Lanciotti16] with minor modifications [Reference Barde17]. To detect viral RNA from mosquitoes, the pooled adult mosquitoes were triturated in 500 μl chilled bovine albumin phosphate saline, centrifuged to remove cell debris and 140 μl of this supernatant was used for RNA extraction and nRT–PCR. For phylogenetic analysis the primers of the envelope non-structural gene junction region described by Kanakaratne et al. [Reference Kanakaratne18] were used. The RT–PCR products were sequenced as described previously [Reference Barde17] and submitted to GenBank. The sequences were compared with 23 sequences of DENV strains from the NCBI database.
Data analyses
The entomological and clinical data were double key-entered in Microsoft Office Excel (Microsoft Corp., USA), validated to avoid entry-level errors, filtered for initial analysis and then further analysed using appropriate statistical tests. The Breteau index (BI), container index (CI), and house index (HI) were calculated using standard formulae [13]. To generate the phylogenetic tree, multiple sequence analysis was performed using CLUSTAL W software. The maximum-likelihood phylogenetic tree, with Kimura two-parameter corrections using E/NS1 gene junction sequences was generated using the MEGA v. 5 tool, with 1000 bootstrap replicates [Reference Tamura19].
RESULTS
Overall, 142 samples from Churcha and 105 from the villages of Narsinghpur district were tested at the Virology Diagnostic Laboratory, Jabalpur, of these 53 (37·3%) and 62 (59%) were found positive for DEN infection, respectively (Table 1). The maximum number of suspected and positive cases (>60%) were from the 15–45 years age group. In Churcha more males were positive compared to females [odds ratio (OR) 2·71, 95% confidence interval (CI) 1·27–5·70, P = 0·0093], whereas in Narsinghpur both sexes had similar risk (OR 0·930, 95% CI 0·496–1·744, P = 0·8223).
Table 1. Age and gender distribution of the suspected and confirmed dengue cases from Korea and Narsinghpur districts of Chhattisgarh and Madhya Pradesh, respectively
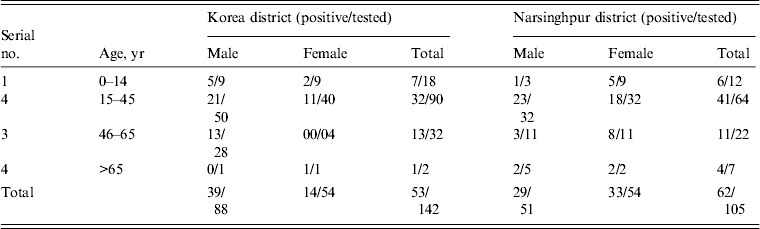
* Positive by NS1 Ag rapid card test, IgM ELISA, and nRT–PCR.
The DEN IgG ELISA conducted on the samples revealed that 87% were negative for IgG and only 6·8% of suspected cases had IgG because of previous DEN infection(s). Ninety-six percent DENV infections were primary and only four cases had secondary infections.
The molecular studies on the samples collected from cases of febrile illness confirmed that the aetiology of both outbreaks was DENV-1, which was the only serotype detected during the outbreaks. CHIK IgM antibodies were not detected in any of the DEN-negative samples.
The clinical investigations in both outbreaks indicated that the cases of febrile illness were DEN, with the most commonly reported symptoms being fever (99%), headache/retro-orbital pain (53%) and joint pain (50%). Few patients (5%) had complaints of rash on the chest, forearms or stomach ache. No case of severe DEN (DHF or DSS) according to the WHO classification was noted. In all, only 19 (17·1%) primary DEN cases required hospitalization. Thrombocytopenia was observed in 17·4% of cases; however, none required platelet transfusion. All four cases with secondary infection had thrombocytopenia.
Adult and immature stages of Aedes species were found in both affected areas. A. aegypti was found in cement tanks, unused utensils and discarded plastic containers indoors. Overhead water tanks, discarded tyres and water accumulated in junk yards were the main breeding areas outside the home. On the day of intervention the HI was 52·40, CI was 26·77 and BI was 100·43, which following implementation of mosquito control measures gradually decreased to 2·05, 0·69 and 2·74, respectively, by day 17 (Fig. 2). Daily data collected in Churcha clearly demonstrated that there was a positive correlation (r = 0·687) between the number of cases and BI. In Churcha colliery, A. albopictus, A. vitatus were also found.

Fig. 2 [colour online]. Graph showing the curve of cases and Breteau index (BI). Cases gradually declined as the BI decreased to <5).
In the villages of Narsinghpur district, only A. aegypti was found. On the day of implementation of mosquito control measures, a BI of 37 and 48 was calculated in Manesur and Imaliya, respectively. The nRT–PCR conducted on the pools of mosquitoes for detection of DENV yielded negative results.
The phylogenetic analyses of the partial and envelope non-structural 1 gene junction (Fig. 3) nucleotide sequences of the virus demonstrated that the virus belonged to genotype III of DENV-1.

Fig. 3. The maximum-likelihood phylogenetic tree with Kimura two-parameter corrections using the E/NS1 gene junction of DENV-1 detected from Churcha, indicated by ▲; DENV-1 from Narshinghpur district indicated by ●. The tree was generated using 23 sequences downloaded from the Genbank database and MEGA v. 5 software. The tree was validated using 1000 bootstraps. The analyses demonstrated that DENV-1 belongs to genotype III.
Follow-up in Churcha colliery in the second week of September revealed that the number of cases had fallen significantly (2/19 IgM positive and no NS1/nRT–PCR positive). Similarly, no cases were detected during December from the villages of Narsinghpur district.
DISCUSSION
India is now endemic/hyperendemic for DEN [Reference Chakravarti, Arora and Luxemburger20, Reference Gupta21]. DENV-3 in 1966 [Reference Rodrigues9] and DENV-2 in 1992 [Reference Mahadev10] were detected from outbreaks in central India. Although only a few sporadic cases and outbreaks have been reported [7, Reference Barde17], the serotypes responsible for outbreaks have not been recorded during the last 20 years in this area.
Different serotypes and their genotypes based on primary or secondary infections are known to exhibit differences in disease severity. Studies have shown that primary infection by DENV-1 results in fairly severe manifestations [Reference Kyle22]. The clinical presentation in both outbreaks was typical of DF, even though the majority of patients had primary infection. The symptoms reported in this outbreak were not severe and <20% of patients required hospitalization or had thrombocytopenia. Two deaths attributed to DEN were brought to the attention of the visiting team in Churcha; however, samples were not available.
Overt disease in adults [Reference Chakravarti, Arora and Luxemburger20] rather than in children, especially in the first infection of DENV-1 is reported earlier [Reference Nishiura23], 90% of the cases in our study were adults (Table 1). It would be worthwhile to evaluate the role of asymptomatic and mild infections in children in DEN epidemiology as they may silently transmit the virus. In several outbreaks from India it was reported that males outnumbered females [Reference Chakravarti, Arora and Luxemburger20]. This was also the case in Churcha as males were at almost double the risk of infection, while in Narsinghpur no significant difference was noted in male/female ratios. These variations in observations could be because of different social, occupational and behavioural practices in these two areas. It was observed in Churcha, that males working in shifts were resting at home in minimal clothing, resulting in their exposure to the diurnal anthropophilic Aedes mosquitoes; however, no such practice was noted in Narsinghpur.
The high density of A. aegypti, the principal vector of DENV, was responsible for the upsurge of DEN cases in both outbreaks. Storage of water in cement tanks, because of the uncertain water supply, and accumulation of rainwater in discarded tyres, etc. provided the breeding areas for the vector mosquitoes, resulting in a high BI. Mosquito control measures mitigated the outbreak and the number of DEN cases decreased as the BI fell (Fig. 2), demonstrating that timely intervention by mosquito control measures could restrain the severity of the outbreak.
It will be interesting to reveal the role of A. albopictus and A. vitatus mosquitoes in the transmission and maintenance of the virus as these species have been found in Korea district previously [Reference Mahadev10] and A. albopictus is known to play an important role in transmission of DENV in other parts of the country [Reference Thenmozhi24].
Different genotypes of DENV serotypes are circulating in India. Although, recently there has been a rise in cases due to DENV-1 [Reference Kukreti25], historically DENV-1 is detected mostly co-circulating with a predominance of other DENV serotypes [Reference Kukreti25]. Although there are reports of DENV-1 circulation from the south (Vellore, Tamil Nadu [Reference Myers26]), north (Delhi [Reference Rao27], Ludhiana, Punjab [Reference Kaur28]) and west (Parbhani, Maharashtra [Reference Mehendale29], Jalore, Rajasthan [Reference Chouhan30]) of India, this is the first outbreak due to DENV-1 in central India. Moreover, no other serotype was detected in the samples tested by nRT–PCR, indicating that the epidemiology of DEN is changing and DENV-1 is probably now emerging as the dominant serotype responsible for outbreaks.
The fast and economical approach of genotyping, based on E/NS1 junction and partial E gene sequences is being employed by several researchers [Reference Kukreti25]. DENV-1 can be categorized into three genotypes using these regions [Reference Kukreti25, Reference Patil31–Reference Hwang33]. Genotype III detected in these outbreaks is known to have circulated persistently in India since 1956, with occasional detection of genotype I in the recent past [Reference Kukreti25].
This study has a few limitations; first, the failure to detect DENV from vectors, probably because larvae were collected instead of fed adults. Second, our inability to identify the index case because of moving populations. Nonetheless, we can conclude that these outbreaks of febrile illness were due to DENV-1 genotype III, which was the only serotype detected. The majority of infections were primary and non-severe. It would be interesting to understand the host and viral factors responsible for this low virulence. Further characterization of DENV-1 by full genome sequencing, using bioinformatics tools, will help to understand aspects of virulence and the relationships of DENV-1 with other DENV.
ACKNOWLEDGEMENTS
The authors are grateful to the Secretary to the Government of India, DHR, MoH & FW, and The Director General, ICMR for financial support under the Viral Diagnostic Network Project and the Directorate of the National Vector Borne Disease Control Programme for providing IgM ELISA kits. The help of doctors and technical staff of the SECL Churcha colliery, Korea and District Hospital, Korea and the technical help of staff of the Virology Department of RMRCT, Jabalpur during the investigation is acknowledged.
DECLARATION OF INTEREST
None.







