Globally in 2011, an estimated 101 million children under 5 years of age were underweight with South Asia having the highest prevalence (33 %) followed by sub-Saharan Africa (21 %)( 1 ). Of this global figure, approximately thirty-three million children under 5 years of age are affected by moderate acute malnutrition (MAM), defined as a weight-for-height z-score between −2 and −3, and at least nineteen million children under 5 by severe acute malnutrition (SAM), defined as a weight-for-height z-score of <−3( Reference Bank 2 , 3 ). For children with SAM, the risk of death is approximately 10-fold higher compared with children with a z-score ≥1( Reference Black, Allen and Bhutta 4 ). High prevalence rates have been reported for low birth weight, protein energy malnutrition, wasting and underweight among children under 5 years of age( Reference Asfaw, Wondaferash and Taha 5 , 6 ). In communities where there are food insecurity challenges, the urgent need to find appropriate solutions that can address these challenges should be recognised. In developing countries where the prevalence of food insecurity is most rife, communities have used various coping strategies to mitigate these challenges as best they could; however, these solutions have hardly minimised the scourged and menace of this condition, particularly among children. A Cochrane review was conducted on specially formulated foods for treating children with MAM in low- and middle-income countries. The review considered eight randomised controlled trials inclusive of among others, lipid-based supplements and different types of blended food and improved adequacy of home diets. The review concluded that lipid-based nutrient supplements and blended foods were effective in treating children with MAM( Reference Lazzerini, Rubert, Pani and Lazzerini 7 ).
Within this decade, several interventions, including both nutrition-specific and nutrition-sensitive programmes, have been adopted over the years by governments and other stakeholders to curb this menace( Reference Bryce, Coitinho and Darnton-Hill 8 – Reference Elver 12 ). Through the Scaling Up Nutrition initiative that came into effect in 2010, the aim was to allow participating countries set out to put together the right policies, collaborated with partners to implement programmes with shared nutrition goals and mobilised resources to effectively scale up nutrition( 13 ). Therapeutic feeding is a nutrition intervention that has a powerful potential to prevent acute malnutrition( Reference Saskia van der, Salse-Ubach and Roll 14 ). Therapeutic feeding, a nutrition-specific intervention, is the use of any appropriate food product or products, enhanced nutritionally and energy-dense for use in emergency situations as this approach allows for humanitarian relief within the shortest time possible( Reference Latham, Jonsson and Sterken 15 ). About twenty million children worldwide are estimated to be suffering from SAM( Reference Ahmed, Hossain and Mahfuz 16 , 17 ) and yet only 10–15% of these SAM children receive treatment using ready-to-use therapeutic food (RUTF)( 17 , Reference Weber, Ryan and Tandon 18 ). A larger percentage of SAM children globally can be found in Asia( Reference Ahmed, Hossain and Mahfuz 16 ) with the highest prevalence in developing countries, particularly in sub-Saharan Africa and South Asia. It can be considered important to compare these developing countries’ efforts to control SAM by their way of RUTF usage to help identify strengths and weakness in therapeutic programme for health policy enhancement.
What is ready-to-use therapeutic food?
RUTF is an energy-dense, mineral-enriched food specifically designed to treat SAM. RUTF has a similar nutrient composition to F100. F100 is a water-soluble ‘catch-up’ formula usually of more energy and protein designed to rebuild wasted tissues in malnourished children. RUTF is a soft, crushable food that can be consumed easily by children from the age of 6 months without adding water. Unlike F100, RUTF is not water-based, meaning that bacteria cannot grow in it and it can be used safely at home without refrigeration and in areas where hygiene conditions are not optimal. It does not require preparation before consumption. Plumpy'nut is an example of a commonly known lipid-based RUTF( Reference Kavita, Doledec and Begum 19 ); however, in this review, its composition will be considered alongside other RUTF.
Application involving the use of ready-to-use-therapeutic food in Africa and outcome
A case study in Ghana, Malawi and Zambia( Reference Maleta and Amadi 20 ) to assess community-based management of acute malnutrition (CMAM) revealed that the main treatment in these three countries was mostly the standard peanut-based RUTF (P-RUTF). It was further identified that although these three countries used the standard P-RUTF, in Ghana and Malawi, RUTF was part of the essential health package and essential drug list that was procured by the Ministry of Health and distributed throughout the health system. Zambia's ministry of health; however, at the time the study was conducted had not developed a budget to take over the responsibility of procuring RUTF for its health system, and so depended on RUTF procured by UNICEF and Clinton Health Access Initiative. Moreover, among the three countries, it is only Malawi that produces RUTF locally and some of their products are being exported to Zambia. A study in Zambia made a comparison between milk-free soya–maize–sorghum-based RUTF and the standard P-RUTF( Reference Irena, Bahwere and Owino 21 ). In identifying the effectiveness of the two types of therapeutic feeds, the study concluded that P-RUTF, which is made up of about 25 % milk content, was more efficient in improving recovery rate, but was expensive to use than the soya–maize–sorghum-based RUTF. This observation makes it a challenge to increase recovery rate of SAM children since more resources must be used to enhance the intervention programme. The challenge of resources is also faced by countries such as Ghana and Malawi( Reference Maleta and Amadi 20 ). It has been argued that local procurement of RUTF among its local suppliers can decrease the cost( Reference Nunez 22 ).
Although the recovery rate of locally manufactured RUTF in Malawi was quiet low compared with the standard P-RUTF, the benefits to the economy were huge. Some of these included job creation, a boost to incomes of local farmers, and shortening of supply chains thereby allowing beneficiaries access to RUTF not only at a cheaper cost but also quicker. The implication for this allowed non-governmental organisations the ease of purchase and distribution of RUTF, which reached the end users timeously( Reference Nunez 22 ).
Evidence of ready-to-use therapeutic food successes
A Delphi process and a systematic review were conducted on the treatment of SAM and MAM in low- and middle-income settings. The review revealed that for in-patient treatment of complicated SAM according to the WHO protocol, a panel of experts estimated case fatality rates of 14 % (range 5–30 %) and recovery rate of 71 % (range 25–95 %)( Reference Lenters, Wazny and Webb 23 ). The lower boundary of the recovery rate of 25 % results from one expert who expressed that a large proportion of admissions would default before recovery was reached. For community-based treatment of SAM, the case fatality rate was estimated at 4 % (range 2–7 %) and a recovery rate was estimated at 80 % (range 50–93 %). The mortality rate for MAM based on optimal treatment proposed by the experts was estimated at 2 % (range 0–4 %) and recovery rate was 84 % (50–100 %)( Reference Lenters, Wazny and Webb 23 ). Furthermore, one review suggests that the case fatality rates tend to be well under 10 % in CMAM programmes( Reference Park, Kim and Ouma 24 ).
Similarly, for community-based treatment of SAM, RUTF were compared with maize–soya blend. Children given RUTF were 51 % more likely to achieve nutritional recovery than the standard care group. For the treatment of MAM, children in the RUTF group were significantly more likely to recover and less likely to be non-responders than in the maize–soya blend group. In both meta-analyses, weight gain in the intervention group was higher( Reference Lenters, Wazny and Webb 23 ).
RUTF was observed to be attractive to donor agencies because of its low cost, consequent ability to increase coverage, as well as greatly reduced burden on local in-patient health care facilities. Thus, by 2011, 3·4 million of cases (1·1 million in DR Congo and 1·6 million in Nigeria alone) were being treated via CMAM in sixty countries. Use of RUTF by UNICEF (the largest user) increased from 200 000 cartons for twenty-seven countries in 2006 to over 1·2 million (300 000 of which were locally produced), equivalent to about 20 000 metric tonnes for over fifty countries in 2010, accompanied by a decrease in use of F100 to about one-third of 2003 levels( Reference Komrska 25 ).
In the quest to know whether locally produced RUTF using locally available ingredients were acceptable to young children in Ethiopia, Ghana, Pakistan and India, a study compared alternative RUTF formula with the standard P-RUTF containing milk powder( Reference Weber, Ryan and Tandon 18 ). The study found out that ingredient cost of all alternative RUTF was about 60 % of the standard RUTF and moreover the local RUTF was tolerated well without increased report of rash, diarrhoea or vomiting in countries such as Ethiopia, Ghana and India. Children in these countries preferred both the local and standard RUTF. The study further observed that in Pakistan, children consumed local RUTF in similar quantities, but their mothers perceived that their children did not enjoy it as much as the standard RUTF. Reasons for this perception of mothers were unknown. A study conducted in Gorory, Sokoto, in northwest Nigeria to determine the effectiveness of 2 weeks nutritional supplementation with RUTF or micronutrient powder to children (6–58 months) diagnosed with and treated for malaria, diarrhoea in reducing the incidence of acute malnutrition found that the short-term supplementations of either RUTF or micronutrient powder did not reduce the incidence of acute malnutrition among the children( Reference Saskia van der, Salse-Ubach and Roll 14 ). However, a similar study in Uganda saw a reduction in incidence of malnutrition in children who received RUTF but not in children who received micronutrient powder( Reference Saskia van der, Salse-Ubach and Roll 26 ). In a study in Tanzania, the percentages of underweight and wasting among HIV children on antiretroviral therapy who did not take RUTF were 12·4 and 16·5 % and where children on antiretroviral therapy took RUTF were 3·0 and 2·8 %, respectively( Reference Sunguya, Poudel and Mlunde 27 ). A qualitative review of an alternative treatment of SAM in Myanmar showed a number of factors that make the usage of RUTF successful( Reference Cosgrove, Earland and James 28 ). The review puts down this success as down to local context, which involves an ideal timing with the absence of natural disasters. Also relevant is community involvement where there is sensitisation, mobilisation and support in handling malnutrition awareness and education, nutrition programming, mother and child that required psycho-social support and home feeding( Reference Cosgrove, Earland and James 28 ).
Challenges with the use of ready-to-use therapeutic foods in the management of malnutrition
Managing acute malnutrition is of high interest to public health especially in low-income countries. However, milk powder is expensive and more often imported. Some children may also show allergenic reaction to the peanut component as well as the zinc ratio in RUTF. Other components of RUTF are imported making the price too high for a poor family to have access( Reference Collins and Henry 29 ). In an attempt to mitigate this challenge, some international organisations have taken advantage to produce improved forms of RUTF of much higher nutrient quality and in commercial quantities with the view of supporting low-income countries( Reference Latham, Jonsson and Sterken 15 ). The danger that these RUTF pose is that they undermine the usual breast-feeding after 6 months through to 24 months. Furthermore, the dependency on the use of RUTF if not carefully promoted can be counterproductive to the children's nutritional status and overall wellbeing( Reference Collins and Henry 29 ). Additionally, children need a lot of water during the day, but unfortunately, RUTF is not known to have water component and thus children being fed on it may likely suffer from dehydration( Reference Latham, Jonsson and Sterken 15 ). In a chronically food insecure area such as southern Ethiopia, RUTF was used and observed to be effective in treating SAM cases, but the challenge that was faced was that caregivers expected RUTF to be supplied for a prolonged period of time instead of the intended short-term provision for the management of SAM cases( Reference Tadesse, Berhane and Hjern 30 ). The study also revealed that mothers and other caregivers saw RUTF as a household commodity to be freely distributed and only sold when there was the need to. This appeared a challenge for the use of RUTF for SAM children in southern Ethiopia. This behaviour could inhibit the success of the therapeutic intervention in chronic food insecure locations. The positive side of the situation was that the people had accepted and knew that RUTF was a high-quality food effective in treating SAM( Reference Tadesse, Berhane and Hjern 30 ).
Food-based approaches to addressing malnutrition among children
Principles governing recipe development include the need for a varied diet to provide essential nutrients; utilising ‘nutrient strengths’ of food ingredients that are locally available and commonly consumed; and utilising local food preparation methods for acceptability of products( Reference Amuna, Zotor and Chinyanga 31 – Reference Zotor, Ellahi and Amuna 33 ). Recipe formulation is also based on normal nutrient requirements for age, plus additional requirements to replace weight deficits assuming energy cost of tissue repletion to be 20·92 kJ/g( Reference Golden, Weatherall, Ledington and Warrell 34 ) resulting from infectious burden, malabsorption, oxidant stress and the high anti-nutrient content of basic plant-based diets as well as requirements for catch-up growth( Reference Golden, Briend and Grellety 35 ). A malnourished 1-year-old weighing 7·5 kg for instance has a weight deficit of 2·5 kg for his age. The extra energy cost of replenishment will therefore be 20·92 kJ × 2500 g = 52 300 kJ (52·13 MJ). This is additional to their normal daily energy requirement. Similarly, in working out protein and micronutrient requirements, a presumption of an extra 25 % of losses (i.e. 2·5 kg weight deficit) serves as a useful guide in calculating nutrient densities and index of nutrient quality for recipes. Furthermore, losses during processing and feeding will have to be taken into account in any formulation. The practical significance of these scientific considerations is relevant at the phase of feeding the individual during nutritional rehabilitation, where a strategy for the attainment of weight gain and replenishment of micronutrients would be adopted and implemented. Increased need for type II nutrients, such as potassium, magnesium and zinc, should also be considered alongside the need to decrease sodium content of the diet (to prevent heart failure) and protein content during the acute phase of treatment (to reduce the metabolic load on the liver), especially in cases of kwashiorkor. These clinical considerations must be central to the principles of recipe formulation for malnourished target groups.
The food multimix concept
Over the past decade and half, the use of the food multimix (FMM) concept has been discussed as an alternative strategy to ensure the most efficient use of the scant food resources to meet energy, protein and micronutrient needs, particularly in vulnerable groups( Reference Amuna, Zotor and Chinyanga 31 – Reference Zotor, Ellahi and Amuna 33 , Reference Zotor, Amuna and Oldewage-Theron 36 – Reference Amuna and Zotor 38 ). In brief, the concept states that irrespective of one's environment, it is possible through the application of nutrition, food science technology, human physiology, biochemistry and metabolism in health and disease (within a social and cultural context) to meet human needs across different population groups. The premise for this concept allows for the effective and efficient use of locally available and commonly consumed but scant food resources within any community.
It has been argued by some researchers including the present authors that a community-based approach to the prevention and management of malnutrition can be safely delivered through a food-based approach employing dietary diversification( Reference Amuna, Zotor and Chinyanga 31 – Reference Zotor, Ellahi and Amuna 33 ). The development of foods targeted at therapeutic and supplementary feeding during nutritional rehabilitation using local food sources should form an important element of the clinical management of the patient.
Admittedly, the advent of RUTF has revolutionised the treatment of children suffering from SAM( Reference Lenters, Wazny and Webb 23 – Reference Komrska 25 ). Indeed, the successes of RUTF in the treatment of MAM and SAM has been very beneficial and timely to reduce the burden of malnutrition, especially among developing countries such as sub-Saharan Africa( Reference Latham, Jonsson and Sterken 15 ). In the short term, integrating the management of acute malnutrition using a CMAM approach into the government's urban and rural community-based health service delivery system, focusing on treating uncomplicated SAM with RUTF and MAM with locally prepared fortified foods, is highly effective and recommended.
Additionally, the metabolic challenges of malnutrition are taken into account in formulating mixes, including meeting energy and protein needs, whilst at the same time, exercising caution in the provision of iron in the diet, especially during the acute phase of treatment. It has been shown that the functional capacity may be exceeded if too much food is given during rehabilitation of the malnourished child. By strictly controlling the energy intake, e.g. by starting with lower strength formulations at the acute phase of treatment, and gradually increasing the intake as the patient improves, nutrient imbalances and unnecessary tissue fluxes can be avoided. It may also be unwise to give high-density products to such patients as it could induce osmotic diarrhoea. Moreover, the gastro-intestinal tract may need time to recover from mucosal damage and secondary malabsorption, and hence not be ready for a high nutrient load at any one time. This therefore calls for formulations that take into account the metabolic considerations inherent in the management of malnutrition. A range of micronutrient-dense foods, mainly from local traditional sources, were selected to ensure familiarity and cultural acceptability whilst maintaining food diversification, at relatively low cost( Reference Zotor and Amuna 32 ). The FMM formulated for nutritional rehabilitating individuals were at different energy densities and nutrient strengths (referred to as lower and higher strengths) based on the WHO ‘Ten Steps’ rationale( Reference Golden, Weatherall, Ledington and Warrell 34 ) and taking into account different nutritional needs of children at different stages of rehabilitation.
In Table 1, the food ingredient of rice, barley and maize-based RUTF were highlighted alongside lower and higher strength FMM recipes. Differences were observed in the dietary diversities (based on nutrient adequacy or the number of different foods or food groups consumed over a given reference period) between the RUTF and the FMM. The proportions of energy-dense foods were higher in the RUTF compared with the FMM. The FMM had a lower strength nutrient formulation to cater for malnourished individuals at an early phase of rehabilitation and then a higher strength, which was meant to cater for individuals at a more stabilised stage of rehabilitation.
Table 1. Food ingredient and percentage compositions of ready-to-use therapeutic food (RUTF) and food multimix (FMM) recipes
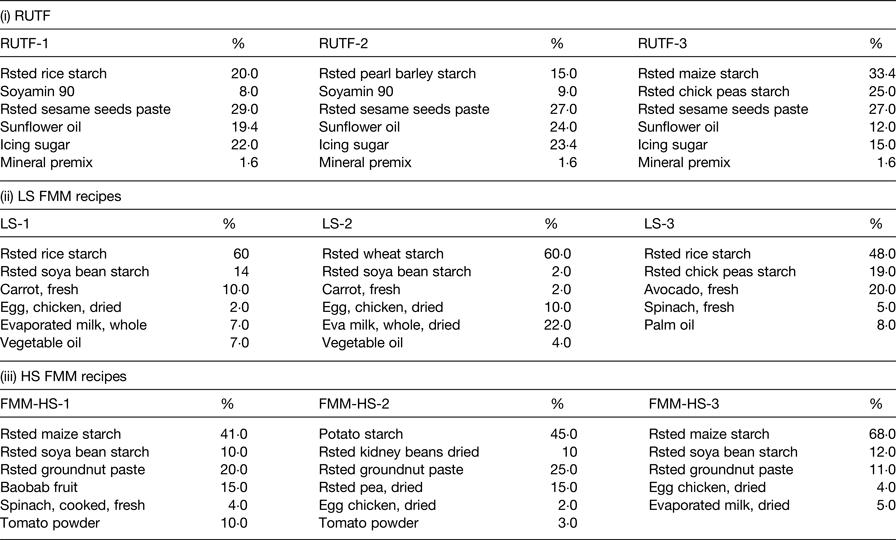
Mineral premix, CMV therapeutic Nutriset; Rsted, roasted; LS, lower strength; HS, higher strength.
Data extracted from Collins & Henry( Reference Collins and Henry 29 ).
In Table 2, the focus was more on the macronutrient composition of RUTF, plumpy'nut and FMM. Energy levels (made up of protein, carbohydrates and fats) for RUTF 1, 2, 3 and plumpy'nut were 2305·38, 2372·32, 2142·20 and 2217·52 kJ, respectively, compared with 1462·72 and 1648·07 kJ for FMM lower and higher strengths, respectively( Reference Zotor, Amuna and Oldewage-Theron 36 ). These energy levels showed clear differences between RUTF, plumpy'nut and FMM. These energy-dense food levels were obvious for the higher ash levels in RUTF compared with FMM. In terms of moisture content, <5 g quantities compared with 26·8 and 15·2 g quantities give an indication that FMM were not in a fully dried state compared with RUTF.
Table 2. Comparison of macronutrient composition of ready-to-use therapeutic food (RUTF) recipes, plumpy'nut® and food multimix (FMM) recipes for nutrition rehabilitation individuals 6–36 months
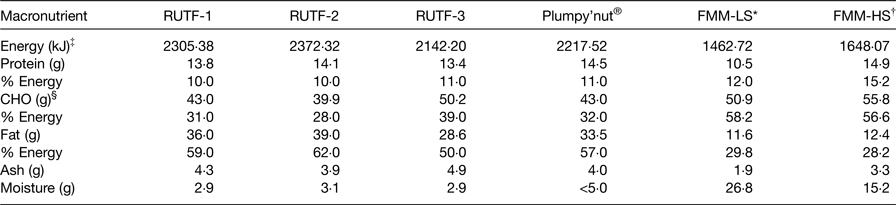
LS, lower strength; HS, higher strength.
* An average LS FMM.
† An average HS; plumpy'nut®: a peanut, sugar, milk powder, vegetable oil and vitamin–mineral mixture.
‡ Energy was calculated using Atwater factors.
§ CHO, carbohydrate calculated by difference assuming protein to be nitrogen (N) times 6·25.
Data extracted from Collins & Henry( Reference Collins and Henry 29 ); Zotor et al. ( Reference Zotor, Amuna and Oldewage-Theron 36 ).
In Table 3, selected mineral contents of RUTF, plumpy'nut and FMM are compared. These theoretical nutrient values are obtained from food databases. Nutrient values for copper were not reported for FMM as these were not available; however, nutrient levels for RUTF 1, 2, 3 were 2·1, 2·1, 1·8 and for plumpy'nut, 1·7 mg/kg, respectively. Zinc nutrient levels were comparable for all three RUTF (an average of 10·7 mg/kg), plumpy'nut (13·0 mg/kg), and for FMM 18·0 and 12·0 mg/kg for lower and higher strengths, respectively. Clear differences were reported for calcium, sodium, magnesium and iron for all RUTF, and plumpy'nut compared with FMM.
Table 3. Comparison of mineral analyses of ready-to-use therapeutic food (RUTF) and food multimix (FMM) recipes for nutrition rehabilitation individuals 6–36 months
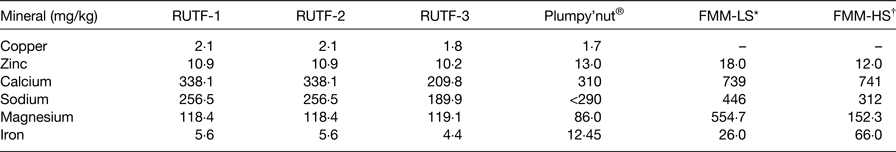
LS, lower strength; HS, higher strength.
* An average LS FMM.
† An average HS; plumpy'nut®: a peanut, sugar, milk powder, vegetable oil and vitamin–mineral mixture.
The iron content in FMM-LS and FMM-HS are largely non-haem and so bioavailability is not as high as with RUTF and plumpy'nut®.
Data extracted from Collins & Henry( Reference Collins and Henry 29 ); Zotor et al. ( Reference Zotor, Amuna and Oldewage-Theron 36 ).
Discussion
We have demonstrated that scant food resources in poor communities can be mixed in ways that would help increase their nutritive value, particularly in areas where food insecurity plays a major role in human health and life expectancy. We proposed that it is possible to meet nutrient needs of vulnerable groups in food insecure communities by harnessing available but scant food resources and combining them in proportions that would provide targeted amounts of daily requirements without the need for fortification. The FMM concept thus takes into account local availability of ingredients, relative costs, cultural factors and above all, clinical or dietetic need( Reference Amuna, Zotor and Chinyanga 31 – Reference Zotor, Ellahi and Amuna 33 , Reference Amuna and Zotor 38 ).
That malnutrition-associated disease is a major cause of death in many poor communities has previously been reported( Reference Golden, Briend and Grellety 35 ). In poor communities, malnutrition is primarily the result of inadequate intake of energy and essential nutrients over time, rather than the result of underlying disease processes per se. This primary process however can lead to secondary (opportunistic) diseases resulting from suppressed immunity, growth failure and unavailability of essential vitamins and minerals, e.g. anti-oxidant nutrients including vitamins A, C and E and minerals such as copper, selenium and zinc that drive the processes of human growth and development.
The design in the formulation holds true based on the fact that decreased energy intake leads to negative energy balance, which presents in the form of poor weight gain in a growing infant or child. Repeated episodes of diarrhoeal disease would in turn, turn a chronic situation in which the individual is developing physiological and biochemical coping mechanisms into an acute state of poor health and high mortality risk. However, the primary goal for a malnourished individual in need of rehabilitation will be to restore lean body mass, promote weight gain and linear growth, restore the efficiency of metabolic processes involved in primary and secondary metabolism and thus reduce the frequency and severity of infections. The latter process requires a well-balanced diet for the improvement in immune function, which can be achieved partly through the use of antioxidant nutrients.
Where there is severe mucosal damage and/or secondary malabsorption, highly concentrated feeds will be rejected leading to osmotic diarrhoea, dehydration and electrolyte imbalance, particularly potassium. FMM recipes allows for this situation to be avoided by developing recipes for feeds of differing strength, starting with the weakest, e.g. lower nutrient-strength, and gradually moving to higher strengths as the patient shows improvement and tolerance of the feeds. In formulating FMM for nutrition rehabilitation, these important clinical considerations were examined and applied.
The argument therefore holds that in the early stages of management of malnourished individuals coming out of the acute phase, if due care and attention is not paid to the nature, type and concentration of formulations, the functional capacity may be exceeded and nutrient imbalances may be aggravated if the individual is given too much food. In considering which recipe to use as food source i.e. whether RUTF or FMM if the child is to rely on it for its total daily requirement, such a diet should contain every essential nutrient in adequate amounts, if the child is to recover properly. The development of FMM recipes takes into account both macro- and micronutrient needs in malnutrition.
Although vitamin A deficiency is a common feature in malnutrition, there are surprisingly many traditional sources of carotenoids and vitamin A, including sweet potatoes, carrots and various green leafy vegetables that are locally available in many developing countries. The therapeutic role of vitamin A can be a key in the management and/or prevention of complications of measles and immune suppression in malnutrition( 39 - Reference Grubesic and Selwyn 41 ). Additionally, good natural food sources of folate in recipes are better recognised by the gastro-intestinal mucosa, and thus are more bioavailable and will promote the methylation and trans-sulphuration cycle and support vitamin B12 metabolism. Folate is also known to be involved in homocysteine metabolism and the formation of glutathione, an important compound in the antioxidant pathways involved in promotion of immune function( Reference Vilaseca, Sierra and Colome 42 ).
Conclusions
This review has demonstrated that it is possible in one composite mix, to provide a food recipe even in the midst of scarcity, to meet nutrient requirements through a food-based approach that is balanced, acceptable and appropriate within a cultural context. Products developed at low cost can be easily available and affordable to most. These FMM can be applied in clinical interventions involving malnourished children who require therapeutic feeding. Although RUTF do have an important role they play in the short-term intervention approach to SAM and CMAM, their likelihood as dependency products does not give room for affected individuals to explore alternate and long-term solutions to healthier recipes.
Acknowledgement
The authors would like to thank the University of Greenwich, UK and the Ghana Health Service for the tremendous help they offered for use of their facilities in formulating the concept and conducting all the analyses and testing the concept without which the present review would not have been possible.
Financial Support
None.
Conflict of Interest
None.
Authorship
F. B. Z. and P. A. developed and designed the FMM concept. F. B. Z. collated results from published and unpublished results, re-analysed and drafted the manuscript with inputs from P. A.; F.B. Z. had primary responsibility for final content. Both authors have critically reviewed and approved the final manuscript.





