Ebstein’s anomaly is a heterogeneous congenital cardiac malformation resulting from failure of tricuspid valve delamination. Most patients who require surgical intervention are able to maintain a biventricular circulation. However, in neonates with an inadequate right ventricle, functional right ventricular outflow tract obstruction, and severe tricuspid regurgitation, the Starnes procedure is an effective initial single-ventricle surgical palliation, with 1- and 10-year survival rates of 87 and 81%, Reference Cleveland and Starnes1 which does not preclude future biventricular repair. Hepatoblastoma is the most common malignant paediatric primary liver tumour Reference López-Terrada2 . It carries a favourable 5-year survival of ∼ 77% Reference López-Terrada2,Reference Kahla, Siegel and Dai3 when treated with chemotherapy and surgical resection. There is one published series of three cases of hepatoblastoma in single-ventricle patients, and one known case of concurrent hepatoblastoma and Ebstein’s anomaly. Reference Windom and Campbell4,Reference Ansell, Mitchell, Roman and Simpson5 We present a novel case of hepatoblastoma in a patient with severe Ebstein’s anomaly following Starnes palliation. This report highlights the patient’s complicated postoperative course and a strategy for managing shunt-dependent physiology alongside malignancy.
Case report
The patient was prenatally diagnosed with severe Ebstein’s anomaly and developed hydrops fetalis during the third trimester. She was delivered at 36 weeks gestation via emergent Caesarean section due to supraventricular tachycardia and fetal distress. Birthweight was 2.68 kilograms with an APGAR score of 6 at 1 minute of life. She was intubated at 4 minutes of life due to hypoxia. Post-natal echocardiography confirmed a Great Ormond Street Echocardiography score 1.16 (Grade 3), Carpentier type C Ebstein’s anomaly with massive right atrial dilation, anatomic pulmonary atresia, and apically displaced tricuspid valve with severe regurgitation (Fig. 1a). Chest X-ray demonstrated a cardiothoracic ratio of 1.0 (Fig. 2a). The patient remained paralysed on invasive positive pressure mechanical ventilation with prostaglandin infusion. She underwent Starnes procedure on day of life 4 with fenestrated Goretex patch right ventricular exclusion, right atrial reduction plasty, ductal ligation, and 3-millimetre central aortopulmonary shunt. She required central post-cardiotomy extracorporeal membrane oxygenator support, with decannulation and sternal closure on postoperative day 4.

Figure 1. a : Echocardiogram on day of life 2, Great Ormond Street Echocardiography score 1.1 (Grade 3). b : Echocardiogram at 11 months of age, Great Ormond Street Echocardiography score 0.4 (Grade 1).
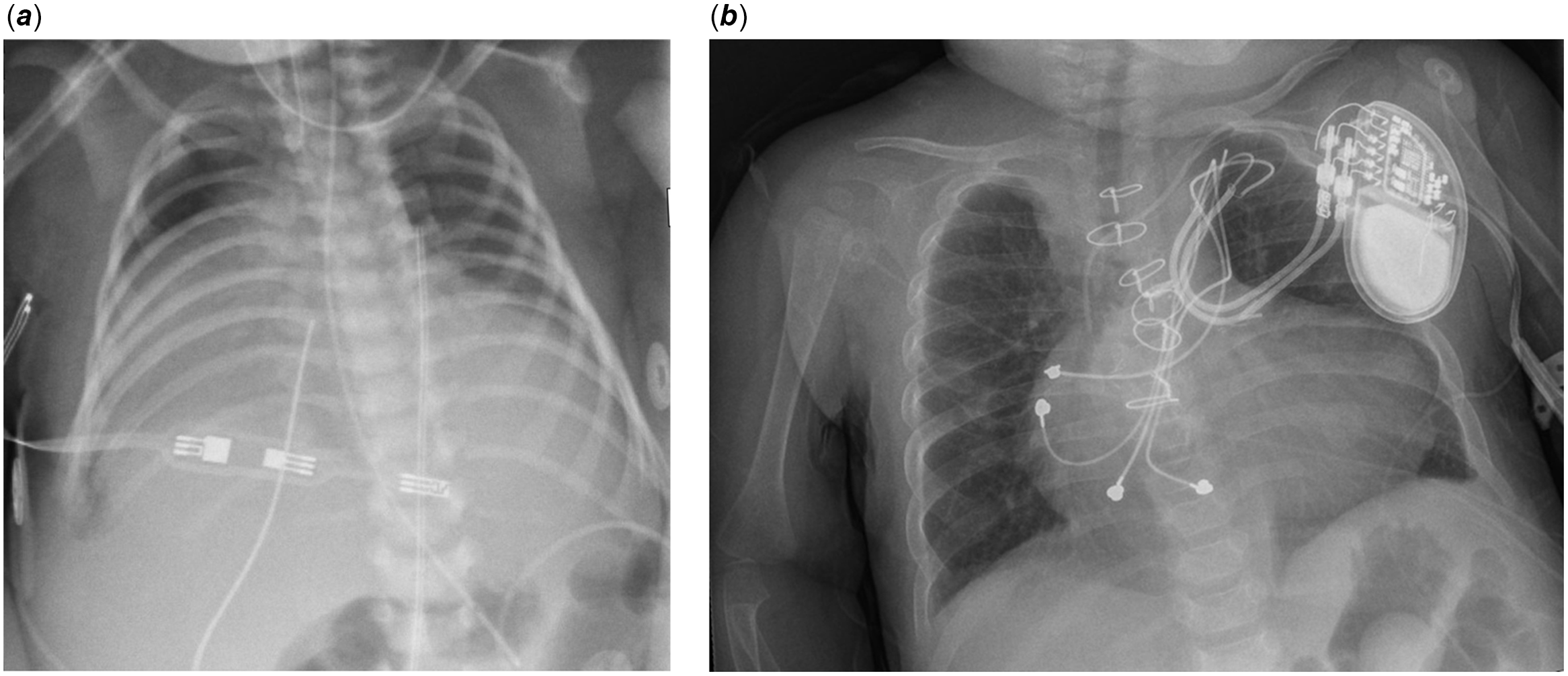
Figure 2. a : Chest X-ray day on of life 2, cardiothoracic ratio 1.0. b : Chest X-ray at 11 months of age, cardiothoracic ratio 0.66.
The patient’s subsequent course was complicated by heart block with episodes of atrial flutter, requiring temporary epicardial pacing and amiodarone. She developed severe capillary leak syndrome, with massive anasarca and large-volume chylothorax, requiring inotropic support, vasopressors, total parenteral nutrition, and repeated antithrombin III, and immunoglobulin replacement. She had multiple episodes of nosocomial infections and sepsis, including rare opportunistic organisms Acinetobacter ursingii and Rhizobium radiobacter. Lymphopenia and immunoglobulinaemia necessitated trimethoprim-sulphamethoxazole prophylaxis with intravenous immunoglobulin replacement. She remained dependent on positive pressure ventilation.
She stabilised two months postoperatively, permitting placement of a dual chamber permanent epicardial pacing system with the generator located in the right upper quadrant of the abdomen. Elevated hepatic transaminases were incidentally noted at three months post-Starnes palliation. Subsequent ultrasound demonstrated an 18 mm central liver mass with associated alpha-fetoprotein of 17,890 nanograms/millilitre. Laparoscopic biopsy demonstrated an epithelial type, embryonal pattern hepatoblastoma. Despite imaging difficulties due to her abdominal generator, complete staging confirmed a PET-avid central hepatoblastoma with additional lesions in the left and right hepatic lobes (PRETEXT Stage III).
In consideration of her shunt-dependent physiology and the extensive hepatectomy required, neoadjuvant chemotherapy with cisplatin was selected for local disease control. Despite adequate hyperhydration, this regimen resulted in acute kidney injury and severe immunosuppression with Candida parapsilosis fungemia, sepsis, and multi-drug resistant pneumonias. These complications delayed subsequent chemotherapy until 5 months of age, when she received three cycles of vincristine and irinotecan. At 8 months of age, she underwent bidirectional cavopulmonary shunt (Glenn procedure) with pacemaker generator relocation to the left deltopectoral region. She developed early stenosis of the cavopulmonary anastomosis with nonocclusive thrombus, requiring thrombectomy and patch plasty 3 weeks postoperatively. Subsequently, her respiratory and renal insufficiency improved dramatically. By 11 months of age, her Great Ormond Street Echocardiography score decreased to 0.4 (Grade 1, Fig. 1b) and cardiothoracic ratio to 0.66 (Fig. 2b). She maintained appropriate local control of her hepatoblastoma, as evidenced by sequential imaging and alpha-fetoprotein levels, and then underwent radiofrequency ablation at an outside institution in lieu of hepatectomy. She remained stable from a cardiorespiratory standpoint and was discharged home on postoperative day 344. She received four additional cycles of outpatient cisplatin and currently remains in remission.
Discussion
We report the first known case of hepatoblastoma in a neonate with severe Ebstein’s anomaly requiring Starnes palliation. Ebstein’s anomaly occurs in approximately 1 per 200,000 live births Reference Attenhofer Jost, Connolly and Dearani6 and hepatoblastoma in approximately 6.58 per million children aged 0-4 years per year nationwide, Reference Kahla, Siegel and Dai3 with their concurrence being exceedingly rare. Individual disease outcomes are generally favourable, but the simultaneous treatment of both presents various challenges requiring detailed, multidisciplinary management.
Great Ormond Street Echocardiography grade 3 Ebstein’s anomaly carries a 10% risk of early mortality and a 45% risk of late mortality; Reference Starnes, Pitlick and Bernstein7 however, both Starnes palliation and early biventricular repair have favourable outcomes. Notwithstanding the hepatic malignancy, this patient had a complicated post-operative course including mechanical circulatory support, prolonged respiratory failure, massive chylothorax, and multiple nosocomial infections, likely due to capillary leak syndrome, diastolic dysfunction, and lack of normal sinus rhythm. The hepatoblastoma diagnosis posed additional challenges. The pacemaker generator location limited initial staging imaging. Standard treatment for PRETEXT III hepatoblastoma is neo-adjuvant platinum-based chemotherapy followed by surgical resection, Reference Chen and Dong8 but her tenuous shunt-dependent physiology rendered extensive hepatectomy too high risk. Acute kidney injury and fungal sepsis complicated cisplatin-based neoadjuvant therapy, requiring second-line chemotherapeutics. Transition to bidirectional cavopulmonary shunt physiology stabilised her cardiac, respiratory, and renal function sufficiently to tolerate radiofrequency ablation and cisplatin-based chemotherapy.
Acknowledgements
The authors thank the patient’s parents for granting permission to publish this case.
Financial support
None.
Competing interests
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the relevant clinical research ethics committee and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the University of Illinois College of Medicine at Peoria Institutional Review Board.





