Refine listing
Actions for selected content:
Contents
Research Article
The Incidence of Cancer of the Bladder and Prostate in Certain Occupations
-
- Published online by Cambridge University Press:
- 15 May 2009, pp. 125-137
-
- Article
-
- You have access
- Export citation
Further Experience of the Bismuth Sulphite Media in the Isolation of Bacillus typhosus and B. paratyphosus B from Faeces, Sewage and Water
-
- Published online by Cambridge University Press:
- 15 May 2009, pp. 138-161
-
- Article
-
- You have access
- Export citation
Lethargic Encephalitis: The Glasgow Epidemic of 1923: Its Incidence and Consequences, from the Point of View of Public Health
-
- Published online by Cambridge University Press:
- 15 May 2009, pp. 162-188
-
- Article
-
- You have access
- Export citation
The Spermicidal Powers of Chemical Contraceptives: II. Pure Substances
-
- Published online by Cambridge University Press:
- 15 May 2009, pp. 189-214
-
- Article
-
- You have access
- Export citation
The Clinical Value of the combined Khan and Wassermann Tests in the Tropics, with Special Reference to Yaws and Syphilis
-
- Published online by Cambridge University Press:
- 15 May 2009, pp. 215-224
-
- Article
-
- You have access
- Export citation
Serological Varieties of Typhus Fever
-
- Published online by Cambridge University Press:
- 15 May 2009, pp. 225-246
-
- Article
-
- You have access
- Export citation
The Examination of the Tissues and Some Observations on the Blood Platelets of Rabbits at Intervals of Five Minutes, and Later, after Intravenous Inoculations of Staphylococcus aureus and Indian Ink
-
- Published online by Cambridge University Press:
- 15 May 2009, pp. 247-256
-
- Article
-
- You have access
- Export citation
Contributions to the Experimental Study of Epidemiology: The Effect of Vaccination on Herd Mortality
-
- Published online by Cambridge University Press:
- 15 May 2009, pp. 257-289
-
- Article
-
- You have access
- Export citation
Front matter
HYG volume 31 issue 2 Cover and Front matter
-
- Published online by Cambridge University Press:
- 15 May 2009, pp. f1-f9
-
- Article
-
- You have access
- Export citation
Back matter
HYG volume 31 issue 2 Cover and Back matter
-
- Published online by Cambridge University Press:
- 15 May 2009, pp. b1-b2
-
- Article
-
- You have access
- Export citation
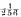 per cent.
per cent.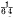 per cent., soaps at
per cent., soaps at 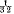 per cent.
per cent.