Cover: An optical image of SiO2-glass microparticles with an average diameter of 98 µm [C.T. Nguyen, K. Koyama, H.T.C. Tu, K. Ohdaira, H. Matsumura: Texture size control by mixing glass microparticles with alkaline solution for crystalline silicon solar cells. p. 1515].
Invited Feature Paper
Texture size control by mixing glass microparticles with alkaline solution for crystalline silicon solar cells
-
- Published online by Cambridge University Press:
- 12 June 2018, pp. 1515-1522
-
- Article
- Export citation
Article
Effect of potential voltages on key functional properties of transparent AZO thin films prepared by electrochemical deposition method for optoelectronic applications
-
- Published online by Cambridge University Press:
- 12 June 2018, pp. 1523-1533
-
- Article
- Export citation
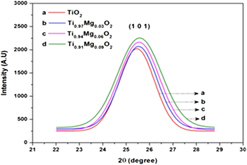
Synthesis of magnesium-doped TiO2 photoelectrodes for dye-sensitized solar cell applications by solvothermal microwave irradiation method
-
- Published online by Cambridge University Press:
- 11 May 2018, pp. 1534-1542
-
- Article
- Export citation
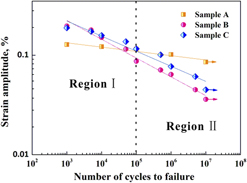
Optimizing fatigue performance of nacre-mimetic PE/TiO2 nanolayered composites by tailoring thickness ratio
-
- Published online by Cambridge University Press:
- 12 June 2018, pp. 1543-1552
-
- Article
- Export citation
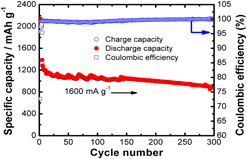
Submicro-sized Si–Ge solid solutions with high capacity and long cyclability for lithium-ion batteries
-
- Published online by Cambridge University Press:
- 25 May 2018, pp. 1553-1564
-
- Article
- Export citation
Invited Paper
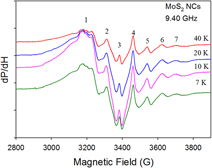
Paramagnetic defects in hydrothermally grown few-layered MoS2 nanocrystals
-
- Published online by Cambridge University Press:
- 12 June 2018, pp. 1565-1572
-
- Article
- Export citation
REVIEW
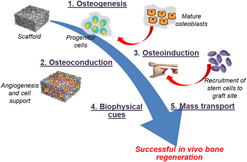
Regenerative medicine: Induced pluripotent stem cells and their benefits on accelerated bone tissue reconstruction using scaffolds
-
- Published online by Cambridge University Press:
- 22 May 2018, pp. 1573-1591
-
- Article
- Export citation
Article
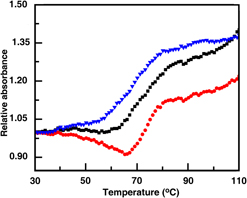
Preliminary study on effect of nano-hydroxyapatite and mesoporous bioactive glass on DNA
-
- Published online by Cambridge University Press:
- 12 June 2018, pp. 1592-1601
-
- Article
- Export citation
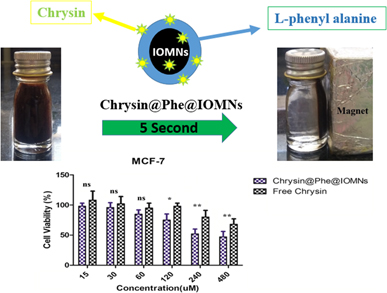
Biocompatibility and anticancer activity of L-phenyl alanine-coated iron oxide magnetic nanoparticles as potential chrysin delivery system
-
- Published online by Cambridge University Press:
- 12 June 2018, pp. 1602-1611
-
- Article
- Export citation
Urea treatment of nitrogen-doped carbon leads to enhanced performance for the oxygen reduction reaction
-
- Published online by Cambridge University Press:
- 12 June 2018, pp. 1612-1624
-
- Article
- Export citation
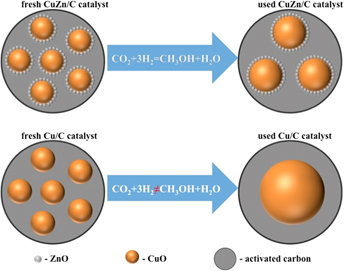
CuO–ZnO anchored on APS modified activated carbon as an enhanced catalyst for methanol synthesis—The role of ZnO
-
- Published online by Cambridge University Press:
- 16 May 2018, pp. 1625-1631
-
- Article
- Export citation
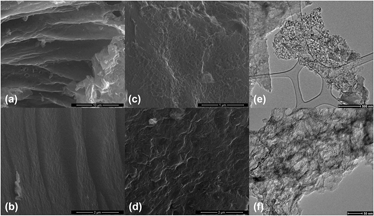
Controllable interlayer shear strength and crystallinity of PEEK components by laser-assisted material extrusion
-
- Published online by Cambridge University Press:
- 21 May 2018, pp. 1632-1641
-
- Article
- Export citation
Experimental analysis and thermodynamic calculations of an additively manufactured functionally graded material of V to Invar 36
-
- Published online by Cambridge University Press:
- 15 May 2018, pp. 1642-1649
-
- Article
- Export citation
Bonding characteristic and electronic property of TiCxN1−x(001)/TiC(001) interface: A first-principles study
-
- Published online by Cambridge University Press:
- 17 April 2018, pp. 1650-1658
-
- Article
- Export citation
Low-temperature synthesis of high-purity boron carbide via an aromatic polymer precursor
-
- Published online by Cambridge University Press:
- 08 May 2018, pp. 1659-1670
-
- Article
- Export citation
Wetting and brazing of Ni-coated WC–8Co cemented carbide using the Cu–19Ni–5Al alloy as the filler metal: Microstructural evolution and joint mechanical properties
-
- Published online by Cambridge University Press:
- 08 May 2018, pp. 1671-1680
-
- Article
- Export citation
Directionally solidified Al2O3/ZrO2 eutectic ceramic prepared with induction heating zone melting
-
- Published online by Cambridge University Press:
- 15 May 2018, pp. 1681-1689
-
- Article
- Export citation
Stabilizing the Tb-based 214 cuprate by partial Pd substitution
-
- Published online by Cambridge University Press:
- 15 May 2018, pp. 1690-1697
-
- Article
- Export citation
Retraction
RETRACTED—Formation and properties of Zr-based bulk quasicrystalline alloys with high strength and good ductility
-
- Published online by Cambridge University Press:
- 25 March 2018, p. 1698
-
- Article
-
- You have access
- HTML
- Export citation
Effect of electropulsing treatment on the microstructure, texture, and mechanical properties of cold-rolled Ti–6Al–4V alloy—RETRACTION
-
- Published online by Cambridge University Press:
- 12 April 2018, p. 1699
-
- Article
-
- You have access
- HTML
- Export citation