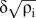Invited Paper
Upconversion rare earth nanoparticles functionalized with folic acid for bioimaging of MCF-7 breast cancer cells
-
- Published online by Cambridge University Press:
- 26 December 2017, pp. 191-200
-
- Article
- Export citation
Article
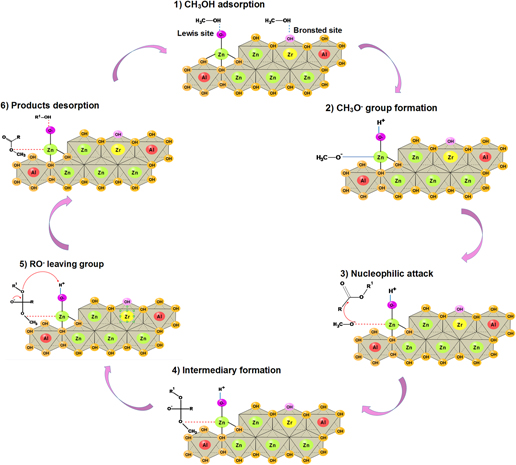
New insights on the basicity of ZnAl–Zr hydrotalcites activated at low temperature and their application in transesterification of soybean oil
-
- Published online by Cambridge University Press:
- 07 September 2018, pp. 3614-3624
-
- Article
- Export citation
Invited Article
CoFe2O4 nanoparticles as efficient bifunctional catalysts applied in Zn–air battery
-
- Published online by Cambridge University Press:
- 30 October 2017, pp. 590-600
-
- Article
-
- You have access
- HTML
- Export citation
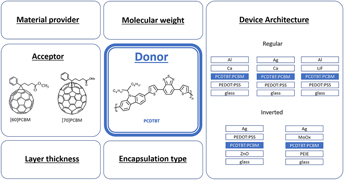
Stability of organic solar cells with PCDTBT donor polymer: An interlaboratory study
-
- Published online by Cambridge University Press:
- 21 June 2018, pp. 1909-1924
-
- Article
- Export citation
Article
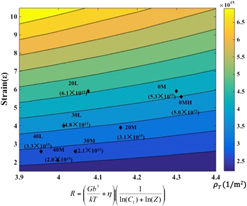
Mapping dislocation densities resulting from severe plastic deformation using large strain machining
-
- Published online by Cambridge University Press:
- 08 August 2018, pp. 3762-3773
-
- Article
- Export citation
Toughening mechanisms of solution-treated SiCp/6061 aluminum matrix composites fabricated via powder thixoforming
-
- Published online by Cambridge University Press:
- 14 August 2018, pp. 2728-2740
-
- Article
- Export citation
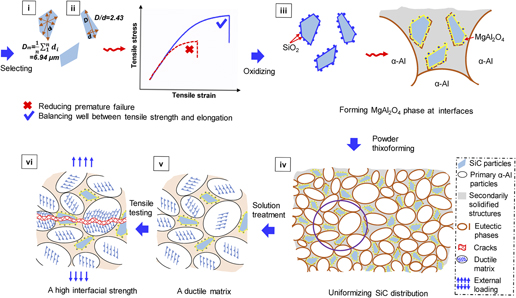
Microstructure of QD-like clusters in GaAs/In(As,Bi) heterosystems
-
- Published online by Cambridge University Press:
- 13 August 2018, pp. 2342-2349
-
- Article
- Export citation
Invited Articles
Indentation response of a 3D non-woven carbon-fibre composite
-
- Published online by Cambridge University Press:
- 16 January 2018, pp. 317-329
-
- Article
- Export citation
Article
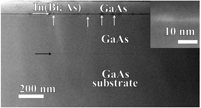
Dilatometric determination of four critical temperatures and phase transition fraction for austenite decomposition in hypo-eutectoid steels using peak separation method
-
- Published online by Cambridge University Press:
- 08 February 2018, pp. 967-977
-
- Article
- Export citation
Erratum
Toward multiscale modeling of thin-film growth processes using SLKMC – ERRATUM
-
- Published online by Cambridge University Press:
- 13 April 2018, p. 872
-
- Article
-
- You have access
- HTML
- Export citation
Article
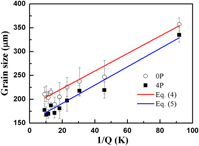
Enhancing grain refinement efficiency and fading resistance of Al–B master alloys processed by equal channel angular pressing
-
- Published online by Cambridge University Press:
- 23 April 2018, pp. 1782-1788
-
- Article
- Export citation
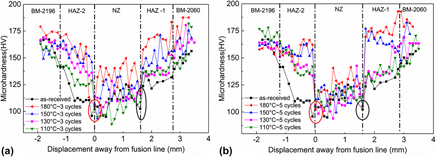
Effect of thermal cycles on the laser beam welded joint of AA2060 alloys
-
- Published online by Cambridge University Press:
- 19 July 2018, pp. 3439-3448
-
- Article
- Export citation
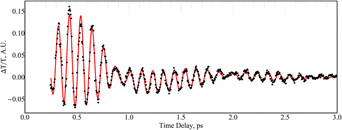
Transient transmission oscillations in doped and undoped lithium niobate induced by near-infrared femtosecond pulses
-
- Published online by Cambridge University Press:
- 09 November 2018, pp. 4207-4214
-
- Article
- Export citation
Articles
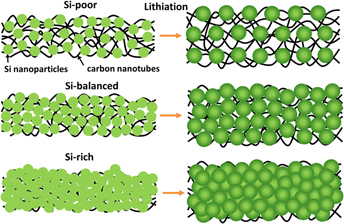
Binder-free freestanding flexible Si nanoparticle–multi-walled carbon nanotube composite paper anodes for high energy Li-ion batteries
-
- Published online by Cambridge University Press:
- 11 January 2018, pp. 482-494
-
- Article
- Export citation
Article
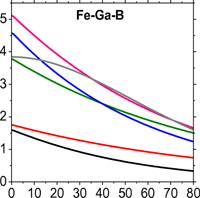
Assessment of Fe–Ga–B alloy magnetomechanical behavior
-
- Published online by Cambridge University Press:
- 12 July 2018, pp. 2207-2213
-
- Article
- Export citation
Front Cover (OFC, IFC) and matter
JMR volume 33 issue 1 Cover and Front matter
-
- Published online by Cambridge University Press:
- 15 January 2018, pp. f1-f4
-
- Article
-
- You have access
- Export citation
Article
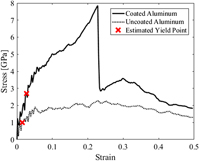
Mechanical enhancement of an aluminum layer by graphene coating
-
- Published online by Cambridge University Press:
- 07 August 2018, pp. 2741-2751
-
- Article
- Export citation
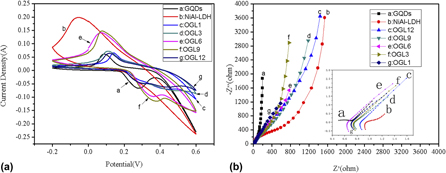
Assembly of Ni–Al layered double hydroxide and oxide graphene quantum dots for supercapacitors
-
- Published online by Cambridge University Press:
- 12 November 2018, pp. 4215-4223
-
- Article
- Export citation
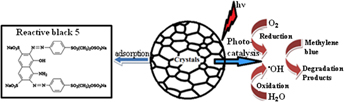
Adsorption and photocatalytic properties of NiO nanoparticles synthesized via a thermal decomposition process
-
- Published online by Cambridge University Press:
- 14 March 2018, pp. 601-610
-
- Article
-
- You have access
- HTML
- Export citation
Articles
The influence of cutting parameters on the defect structure of subsurface in orthogonal cutting of titanium alloy
-
- Published online by Cambridge University Press:
- 17 October 2017, pp. 720-732
-
- Article
- Export citation