74 results
Association of dietary manganese intake and the IL1R1 rs3917225 polymorphism with thyroid cancer risk: a prospective cohort study in Korea
-
- Journal:
- British Journal of Nutrition / Volume 132 / Issue 12 / 28 December 2024
- Published online by Cambridge University Press:
- 13 November 2024, pp. 1584-1592
- Print publication:
- 28 December 2024
-
- Article
-
- You have access
- HTML
- Export citation
Role of Manganese in the Oxidation of Arsenite by Freshwater Lake Sediments
-
- Journal:
- Clays and Clay Minerals / Volume 29 / Issue 3 / June 1981
- Published online by Cambridge University Press:
- 01 July 2024, pp. 219-225
-
- Article
-
- You have access
- Export citation
Manganese Minerals in Clays: A Review
-
- Journal:
- Clays and Clay Minerals / Volume 28 / Issue 5 / October 1980
- Published online by Cambridge University Press:
- 01 July 2024, pp. 346-354
-
- Article
-
- You have access
- Export citation
Influence of Mn2+ and pH on the Formation of Iron Oxides from Ferrous Chloride and Ferrous Sulfate Solutions
-
- Journal:
- Clays and Clay Minerals / Volume 37 / Issue 5 / October 1989
- Published online by Cambridge University Press:
- 02 April 2024, pp. 451-458
-
- Article
-
- You have access
- Export citation
Refinement of Mn-Substituted Muscovite and Phlogopite
-
- Journal:
- Clays and Clay Minerals / Volume 34 / Issue 1 / February 1986
- Published online by Cambridge University Press:
- 02 April 2024, pp. 7-16
-
- Article
-
- You have access
- Export citation
Influence of Manganese Oxide Minerals on the Formation of Iron Oxides
-
- Journal:
- Clays and Clay Minerals / Volume 36 / Issue 5 / October 1988
- Published online by Cambridge University Press:
- 02 April 2024, pp. 467-475
-
- Article
-
- You have access
- Export citation
High-Resolution Transmission Electron Microscopy Study of Mn-Oxyhydroxide Transformations and Accompanying Phases in a Lateritic Profile of Moanda, Gabon
-
- Journal:
- Clays and Clay Minerals / Volume 39 / Issue 3 / June 1991
- Published online by Cambridge University Press:
- 02 April 2024, pp. 254-263
-
- Article
-
- You have access
- Export citation
Oxidation of 1,2- and 1,4-Dihydroxybenzene by Birnessite in Acidic Aqueous Suspension
-
- Journal:
- Clays and Clay Minerals / Volume 37 / Issue 5 / October 1989
- Published online by Cambridge University Press:
- 02 April 2024, pp. 479-486
-
- Article
-
- You have access
- Export citation
Scanning Electron Microscopic and X-ray Powder Diffraction Study of Manganiferous Bauxite, Kincsesbánya, Hungary
-
- Journal:
- Clays and Clay Minerals / Volume 33 / Issue 6 / December 1985
- Published online by Cambridge University Press:
- 02 April 2024, pp. 532-538
-
- Article
-
- You have access
- Export citation
Characterization of Goethite and Hematite in a Tunisian Soil Profile By Mössbauer Spectroscopy
-
- Journal:
- Clays and Clay Minerals / Volume 34 / Issue 3 / June 1986
- Published online by Cambridge University Press:
- 02 April 2024, pp. 275-280
-
- Article
-
- You have access
- Export citation
Electron Transfer Processes Between Hydroquinone and Hausmannite (Mn3O4)
-
- Journal:
- Clays and Clay Minerals / Volume 36 / Issue 4 / August 1988
- Published online by Cambridge University Press:
- 02 April 2024, pp. 297-302
-
- Article
-
- You have access
- Export citation
Transformation of Akaganéite Into Goethite and Hematite in the Presence of Mn
-
- Journal:
- Clays and Clay Minerals / Volume 39 / Issue 2 / April 1991
- Published online by Cambridge University Press:
- 02 April 2024, pp. 144-150
-
- Article
-
- You have access
- Export citation
Effect of Cysteine and Manganese on the Crystallization of Noncrystalline Iron(III) Hydroxide at pH 8
-
- Journal:
- Clays and Clay Minerals / Volume 38 / Issue 1 / February 1990
- Published online by Cambridge University Press:
- 02 April 2024, pp. 21-28
-
- Article
-
- You have access
- Export citation
Mn-Substituted Goethite and Fe-Substituted Groutite Synthesized At Acid pH1
-
- Journal:
- Clays and Clay Minerals / Volume 37 / Issue 2 / April 1989
- Published online by Cambridge University Press:
- 02 April 2024, pp. 151-156
-
- Article
-
- You have access
- Export citation
Weathering Sequence and Alteration Products in the Genesis of the Graskop Manganese Residua, Republic of South Africa
-
- Journal:
- Clays and Clay Minerals / Volume 36 / Issue 5 / October 1988
- Published online by Cambridge University Press:
- 02 April 2024, pp. 448-454
-
- Article
-
- You have access
- Export citation
Effect of Manganese on the Transformation of Ferrihydrite into Goethite and Jacobsite in Alkaline Media
-
- Journal:
- Clays and Clay Minerals / Volume 35 / Issue 1 / February 1987
- Published online by Cambridge University Press:
- 02 April 2024, pp. 11-20
-
- Article
-
- You have access
- Export citation
Transformation of Hausmannite into Birnessite in Alkaline Media
-
- Journal:
- Clays and Clay Minerals / Volume 36 / Issue 3 / June 1988
- Published online by Cambridge University Press:
- 02 April 2024, pp. 249-257
-
- Article
-
- You have access
- Export citation
Oxidation of Dihydroxybenzenes in Aerated Aqueous Suspensions of Birnessite
-
- Journal:
- Clays and Clay Minerals / Volume 37 / Issue 4 / August 1989
- Published online by Cambridge University Press:
- 02 April 2024, pp. 341-347
-
- Article
-
- You have access
- Export citation
Melt synthesis and characterization of synthetic Mn-rich tainiolite
-
- Journal:
- Clays and Clay Minerals / Volume 57 / Issue 2 / April 2009
- Published online by Cambridge University Press:
- 01 January 2024, pp. 271-277
-
- Article
-
- You have access
- Export citation
An Electron Paramagnetic Resonance Spectroscopy Investigation of the Retention Mechanisms of Mn and Cu in the Nanopore Channels of Three Zeolite Minerals
-
- Journal:
- Clays and Clay Minerals / Volume 60 / Issue 6 / December 2012
- Published online by Cambridge University Press:
- 01 January 2024, pp. 588-598
-
- Article
-
- You have access
- Export citation

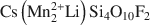 , was synthesized by a high-temperature melt-synthesis technique. Subsequent annealing for 10 days led to a single-phase and coarsegrained material. Single-crystal X-ray diffraction studies were performed and characteristic geometric parameters were compared to the analogous ferrous compound, synthetic Fe-rich tainiolite,
, was synthesized by a high-temperature melt-synthesis technique. Subsequent annealing for 10 days led to a single-phase and coarsegrained material. Single-crystal X-ray diffraction studies were performed and characteristic geometric parameters were compared to the analogous ferrous compound, synthetic Fe-rich tainiolite, 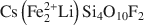 . Both tainiolite structures are outside the compositional stability limits for the 2:1 layer structure, and incorporating the relatively large cation Mn
. Both tainiolite structures are outside the compositional stability limits for the 2:1 layer structure, and incorporating the relatively large cation Mn